the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The Bouraké semi-enclosed lagoon (New Caledonia) – a natural laboratory to study the lifelong adaptation of a coral reef ecosystem to extreme environmental conditions
Federica Maggioni
Mireille Pujo-Pay
Jérome Aucan
Carlo Cerrano
Barbara Calcinai
Claude Payri
Francesca Benzoni
Yves Letourneur
Riccardo Rodolfo-Metalpa
According to current experimental evidence, coral reefs could disappear within the century if CO2 emissions remain unabated. However, recent discoveries of diverse and high cover reefs that already live under extreme conditions suggest that some corals might thrive well under hot, high-pCO2, and deoxygenated seawater. Volcanic CO2 vents, semi-enclosed lagoons, and mangrove estuaries are unique study sites where one or more ecologically relevant parameters for life in the oceans are close to or even worse than currently projected for the year 2100. Although they do not perfectly mimic future conditions, these natural laboratories offer unique opportunities to explore the mechanisms that reef species could use to keep pace with climate change. To achieve this, it is essential to characterize their environment as a whole and accurately consider all possible environmental factors that may differ from what is expected in the future, possibly altering the ecosystem response.
This study focuses on the semi-enclosed lagoon of Bouraké (New Caledonia, southwest Pacific Ocean) where a healthy reef ecosystem thrives in warm, acidified, and deoxygenated water. We used a multi-scale approach to characterize the main physical-chemical parameters and mapped the benthic community composition (i.e., corals, sponges, and macroalgae). The data revealed that most physical and chemical parameters are regulated by the tide, strongly fluctuate three to four times a day, and are entirely predictable. The seawater pH and dissolved oxygen decrease during falling tide and reach extreme low values at low tide (7.2 pHT and 1.9 mg O2 L−1 at Bouraké vs. 7.9 pHT and 5.5 mg O2 L−1 at reference reefs). Dissolved oxygen, temperature, and pH fluctuate according to the tide by up to 4.91 mg O2 L−1, 6.50 ∘C, and 0.69 pHT units on a single day. Furthermore, the concentration of most of the chemical parameters was 1 to 5 times higher at the Bouraké lagoon, particularly for organic and inorganic carbon and nitrogen but also for some nutrients, notably silicates. Surprisingly, despite extreme environmental conditions and altered seawater chemical composition measured at Bouraké, our results reveal a diverse and high cover community of macroalgae, sponges, and corals accounting for 28, 11, and 66 species, respectively. Both environmental variability and nutrient imbalance might contribute to their survival under such extreme environmental conditions. We describe the natural dynamics of the Bouraké ecosystem and its relevance as a natural laboratory to investigate the benthic organism's adaptive responses to multiple extreme environmental conditions.
- Article
(9772 KB) - Full-text XML
-
Supplement
(1075 KB) - BibTeX
- EndNote
Atmospheric carbon dioxide (CO2) has steadily increased over the industrial period (Gattuso et al., 2015), leading to ocean warming, acidification, and deoxygenation. Although the extent to which these stressors will affect marine life is still debated, there is no doubt that their combination will negatively affect a range of marine organisms (e.g., Kroeker et al., 2011; Wittmann and Pörtner, 2013; Hughes et al., 2018). Coral reefs are among the most productive and biodiverse marine ecosystems on Earth. Their survival is expected to be compromised by climate change, whose impacts on reef structures and associated communities span from biodiversity loss to ecosystem shift (e.g., Fabricius et al., 2013; Sunday et al., 2017; Agostini et al., 2018).
Marginal and extreme environments, where some species persist under suboptimal environmental conditions, have become a precious tool to investigate the potential resilience of marine organisms in the face of climate change (Camp et al., 2017, 2018). These sites may be used as natural laboratories where at least one or more environmental parameters naturally mimic extreme environmental conditions over a large area of the ecosystem. They provide an opportunity to simultaneously investigate changes in species responses and their ability to acclimatize and adapt to global environmental changes (Soares, 2020; Kurihara et al., 2020). Shallow-water volcanic CO2 seeps, low pH springs, semi-enclosed bays, mangrove habitats, shallow sheltered-bay reefs, macrotidal environments, and low-pH upwelling areas are all potential study systems where the surrounding seawater is subject to a localized or widespread increase in either pCO2 or temperature and eventually a decrease in dissolved oxygen (DO; Camp et al., 2018). At these sites, general observations suggest ecosystem-level consequences of lifelong exposure to extreme conditions, such as reduced biological diversity, especially among calcifying organisms, decreased rates of coral calcification, and high rates of bioerosion (e.g., Hall-Spencer et al., 2008; Manzello et al., 2008; Fabricius et al., 2011; Crook et al., 2013; Kroeker et al., 2011; Iglesias-Prieto et al., 2014; Milazzo et al., 2014; Paytan et al., 2014). However, some natural laboratories can host very rich reef communities. Examples of such sites have been documented in Palau (Golbuu et al., 2016; Barkley et al., 2017; Shamberger et al., 2018; Kurihara et al., 2021), Papua New Guinea (Pichler et al., 2019), the Kimberley region, Australia (Dandan et al., 2015; Schoepf et al., 2015), mangrove lagoons of New Caledonia (Camp et al., 2017), and the US Virgin Islands (Yates et al., 2014). These natural laboratories have become a common experimental asset in climate change research. However, the lack of empirical characterization of the physical and biogeochemical conditions, including diurnal and seasonal fluctuations, have been argued to bias the interpretation of the biological mechanisms that trigger the responses of organisms (e.g., Vizzini et al., 2013; Camp et al., 2018; Aiuppa et al., 2021). Using limited environmental descriptors makes it difficult to unequivocally identify the main driver(s) of the biological response among the primary factors (i.e., acidification, warming, and/or deoxygenation), the potential secondary factors (e.g., pollution, water flow, tide, seawater nutrient and organic content, turbidity, etc.), and their combination. For instance, at CO2 seeps, pH variability can unexpectedly go beyond projected future values (e.g., Hall-Spencer et al., 2008; Kerrison et al., 2011), and the potential emission of toxic compounds, such as sulfur, arsenic, and metal trace elements (Vizzini et al., 2013), compromises the attribution of specific responses to ocean acidification. Water temperature, pH, and dissolved oxygen can also co-vary negatively or positively and combine with other secondary factors, acting synergistically or antagonistically with unknown effects on benthic community responses. Their extreme values and the extent to which organisms are exposed are crucial in shaping biological responses (Boyd et al., 2016; Rivest et al., 2017). For instance, early studies suggest that temperature fluctuations due to diel or tidal variations could expose corals to stressful temperatures long enough to induce acclimatization or adaptation but short enough to avoid coral mortality (Craig et al., 2001; Oliver and Palumbi, 2011; Castillo et al., 2012; Palumbi et al., 2014; DeCarlo et al., 2019). Coral reef organisms from such thermally variable environments are expected to respond positively to future heat events (Rivest et al., 2017). Besides, corals naturally subjected to high pCO2 variability have shown immune defenses when experimentally exposed to high temperatures, thus buffering the magnitude of thermal stress during heat waves (Wall et al., 2021). The extreme tidal range in the Kimberley region (northwest of Australia) exposes corals to short-term temperature maxima of up to 37 ∘C and fluctuations of up to 7 ∘C daily. Despite the high temperature, also combined with strong currents and turbid waters, diverse and probably resilient coral reefs have been described there (Dandan et al., 2015; Schoepf et al., 2015).
Overall, only parts of these natural laboratories' physical and biogeochemical parameters have been quantified and only during short periods of fieldwork due to logistic constraints. Long-term monitoring of seawater parameters and their fluctuation is essential to better understand the mechanisms used by resilient reef organisms in these natural laboratories and interpret how marine taxa will respond to future biogeochemical changes in the environment.
The semi-enclosed lagoon of Bouraké (New Caledonia, southwest Pacific Ocean) has been considered one of the most suitable natural laboratories for future extreme environmental conditions (Camp et al., 2019). In a preliminary study, Camp et al. (2017) reported a series of compelling short-term data of seawater carbonate chemistry and a general survey of the coral populations. In this first assessment of the Bouraké lagoon, the authors measured diel fluctuations of temperature (ranging from 25.9 to 33.1 ∘C), pH (ranging from 7.24 to 7.91 pHT units) and DO (ranging from 1.80 to 6.97 mg L−1) regulated by a 1 m tide. They revealed that about 20 species of corals were exposed 44 % of the time to a pHT of 7.7–7.8 and 71 % of the time to temperatures predicted for the end of the century under the Intergovernmental Panel on Climate Change (IPCC) scenario RCP4.5 (IPCC, 2014). These striking preliminary findings qualified the Bouraké lagoon as a unique site where potentially adapted corals withstand extreme environmental conditions.
The extent to which the Bouraké species are exposed to suboptimal conditions remains unclear. We believe that the best way to assess these very encouraging findings is to start with fully characterizing the main environmental parameters and the daily and seasonal fluctuations to which reef species have been subjected during their entire life. Here we used a multi-scale approach to map and describe the benthic community living in the Bouraké lagoon and report on new evidence based on 3 years of data. Because of the size of the area, the proximity of a dense mangrove forest, and the demonstrated tide effect on the local environmental conditions, we hypothesize that (1) environmental conditions fluctuate regularly but are spatially heterogeneous throughout the Bouraké lagoon and (2) only a limited number of species can resist the extreme physical and chemical conditions at the study site when compared to adjacent bay-sheltered reefs.
2.1 Study sites
Starting in February 2016, we studied the semi-enclosed coral reef lagoon of Bouraké (South Province, Grande Terre, New Caledonia) and adjacent reference reefs (Fig. 1). The mangrove forest of Bouraké is located in a semi-arid geographical area, and it lacks river input typical of mangrove estuaries.
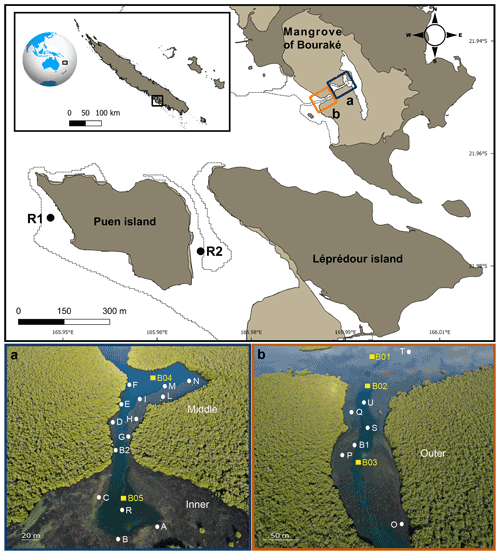
Figure 1Map of the study site (top panel) showing the semi-enclosed mangrove lagoon of Bouraké and reference reefs (R1 and R2). Photos (a) and (b) are aerial pictures (taken at 130 m above the Bouraké lagoon) of the inner (bottom) and middle reefs (a) and the outer reef (b). White dots and yellow squares indicate the sampling stations used for physical and chemical environmental monitoring, respectively. Georep New Caledonian database (https://georep.nc/, last access: January 2021) and QGis software were used to build the figure (top panel).
In Bouraké, a channel of more than 80 m wide and 0.5 to 6 m deep penetrates a dense mangrove forest made up of Avicennia marina and Rhizophora stylosa. It expands into side pools and a large reservoir in the inner part of the mangrove system. A preliminary calculation of the surface and seawater volume of the main area (i.e., without the large seawater reservoir covered by the mangrove forest) using the 3ds Max Model gives underestimated values of 192 100 m2 and 246 060 m3, respectively. Terraces extend from the mangrove forest on both sides of the channel and form diverse and compact reefs at their ends. Coral species are less abundant and diverse near the mangrove roots, where the bottom substrate is composed of fine sediment and mud. There, reefs are exposed to air only at low tide during the spring tides (1.1 m maximum tidal range). We subdivided the lagoon into three main areas: an external, an intermediate, and an inner reef (hereafter called outer, middle, and inner reefs; Fig. 1a and b). A series of sampling stations were selected, and some of the main seawater chemical and physical environmental parameters were measured at the study site and two adjacent reference reefs, namely R1, a typical fringing reef, and R2, a semi-enclosed shallow and relatively large bay. Both reefs are located 4.8 and 3.2 km from the entrance of the Bouraké lagoon, respectively.
2.2 Environmental monitoring
2.2.1 Oceanographic sensors deployment and short- to long-term measurements
From February 2016 to December 2020 up to eight YSI 600 OMS-M loggers, three Seabird SeaFETTM pH loggers, and four Hobo Water Temperature Pro V2 loggers were set at 10 min logging intervals and used individually or in combination to assess short-, medium-, or long-term variations across depth (as a proxy of the tide), temperature (∘C), dissolved oxygen concentration (DO, mg L−1), seawater pHT (total scale), and salinity (see Table S1 in the Supplement for a summary of data collected during the duration of this study). YSI dissolved oxygen optical sensors were calibrated against zero and 100 % saturated seawater at 25 ∘C. Two SeaFETs were calibrated by the manufacturer, while the third was corrected before deployment by measuring its deviation from the two others in the same seawater.
Short-term (i.e., 24 h) spatial and depth-related variations in pH and DO were simultaneously measured at several stations in the Bouraké lagoon in March and April 2018 (Fig. 1). Spatial variations were assessed (i) within the inner (Stations, Sts, A–C for pH and A–G for DO), the middle (Sts H–N for DO), and the outer (Sts O–Q for DO) reefs, (ii) between the outer and the inner reefs (Sts B1 and B2 for pH), and (iii) between the outer and the reef outside the semi-enclosed lagoon (St U vs. T for pH). In the Bouraké lagoon, we recorded differences between the surface (shallow) and the bottom water: (i) at the outer reef (St S for pH), (ii) at the middle reef (Sts I and N for DO), and (iii) at the inner reef (St R for pH and DO). Salinity was measured in July 2019 (Sts R1, R2, B1, and B2) and December 2020 (Sts R1, R2, T, and B2).
Medium-term measurements (i.e., 2–3 weeks) of the DO and pH were recorded at the reference (Sts R1 and R2) and Bouraké reefs (Sts B1 and B2) between 2016 and 2019.
Long-term measurements (>1 year) of seawater temperature were recorded at R1 and R2 starting from January 2019 and September 2017, respectively, and at B1 and B2 from October 2018 and September 2017, respectively. Only temperature data between October 2018 and April 2020 were compared between sites.
Short-term pH and DO data and long-term temperature data were compared between stations using general linear modeling (GLM), and the Tukey' HSD (honestly significant difference) post hoc test was used when significant factor effects were found. When data did not conform to normality or homogeneity of variance, the Kruskal–Wallis test followed by the Dunn's multiple comparisons test (Bonferroni-adjusted) or the Wilcoxon test were performed. Statistical analyses were carried out using either Statistica® or R version 3.4.4 (R Core Team, 2018), the latter using “stats”, “FSA”, and “MASS” packages.
2.2.2 Phase-averaged and tidal harmonic analyses for diurnal and semidiurnal oscillations
Medium-term pH and DO changes were investigated by averaging time and tidal phases for diurnal and semi-diurnal oscillations. To do this, all data were overlaid on a daily period and a tidal phase. First, we calculated a predicted tide for the study area using the Nouméa harbor tide (50 km south of our study site) modified with coefficients from the Naval Hydrographic and Oceanographic Service (SHOM; http://data.shom.fr, last access: February 2021). The predicted tide was used to assign a semidiurnal tidal phase (12 h) to each sampling time, and the data were averaged for each of these tidal phases. Similarly, the data were averaged for each hour of the day (24 h). Because tides at sea are a sequence of sinusoidal harmonic components that are different for each location, we performed a harmonic tidal analysis on the DO and pH data. We used the “UTide”-ut_solv tidal analysis package (Codiga, 2011) with the principal semidiurnal lunar constituent (M2), principal semidiurnal solar constituent (S2), and solar diurnal constituent (S1). For each parameter, the amplitudes of the tidal harmonics M2 (12.4 h), S2 (12 h), and S1 (24 h) were calculated with a 95 % confidence interval based on the 200 Monte-Carlo simulations.
2.2.3 Diel cycles of carbonate chemistry and chemical parameters
Surface water samples were collected across a diel cycle in June 2017 and July 2019 for pH, total alkalinity (AT), dissolved inorganic carbon (DIC), nutrients (orthosilicic acid [Si(OH)4], nitrogen oxide [NOx] (i.e., the sum of and ), ammonium [NH4]+, phosphate [PO4]3−), dissolved organic carbon (DOC), particulate organic carbon (POC), and particulate organic nitrogen (PON) (see Table S2 for a summary of data collected). Dissolved inorganic nitrogen (DIN) and total organic carbon (TOC) were calculated as and DOC+POC, respectively. The most important ratios were calculated (e.g., DOC:TOC; Si(OH)4:DIN) and contrasted between stations to evaluate the availability of nutrients and organic matter in the Bouraké lagoon (Jacquet et al., 2006; Leopold et al., 2017).
In 2017, during 3 consecutive days (from 31 May to 2 June), seawater was sampled six times: twice during both high and low tides and once at both rising and falling tides. In total, we sampled one reference station (R2), three stations at the outer reef of the Bouraké lagoon (outer: Sts B01–B03), one at the middle reef (middle: St B04), and one at the inner reef inside the lagoon (inner: St B05) (Fig. 1). The whole collection lasted about 30 min.
In 2019, during 3 consecutive days (from 16 to 18 July), sampling was carried out every hour from 08:00 to 15:00 LT. We sampled B1 and B2 on the first day, R1 on the second day, and R2 on the third day.
During diel cycles, at each station and sampling time, pH and temperature were measured at the surface (0.5 m deep) using a portable pH meter (913, Metrohm) calibrated with tris buffer (Dickson Lab, batch no. T28). A subsample (50 mL) was filtered through 0.45 µm WhatmanTM Puradisc CA filters using a syringe and poisoned with 20 µL saturated HgCl2 to further measure AT. Two 20 mL subsamples were analyzed using an auto titrator (Eco Titrator, Metrohm), and AT was calculated from the Gran function. Results were corrected against AT standards (Andrew G. Dickson, batch no. 155, Scripps, USA). The seawater carbonate parameters pCO2, , and aragonite saturation state (Ωara) were then calculated from the pHT, AT, temperature, and mean salinity (35) using the free-access CO2SYS package (Pierrot et al., 2006).
Ammonium concentration was determined on a 40 mL subsample of unfiltered seawater, collected using a 60 mL Schott bottle, and stored in the dark. Samples were processed using a fluorimeter (Turner Designs) between 6 and 18 h after 2 mL of OPA reagent (o-phthaldialdehyde) was added (Holmes et al., 1999).
The sampling of nutrients was performed using two replicate 20 mL polypropylene vials, rinsed three times using filtered seawater (WhatmanTM Puradisc CA syringe filters 0.45 µm), filled with the sample, and immediately poisoned with 20 µL saturated HgCl2. Measurements of , NOx, and Si(OH)4 nutrients were performed by colorimetry (Seal Analytical).
Seawater samples for DIC were collected in two replicate glass vials (20 mL), filled with unfiltered water, and poisoned with 10 µL saturated HgCl2. The vials were immediately closed, the absence of bubbles was visually checked, and the samples were stored in the dark at room temperature for later analysis on a Shimadzu TOC-L analyzer (non-dispersive infrared, NDIR). Typical analytical precision was less than ± 2 µmol kg−1. The accuracy was verified using regular measurements of reference material (CRM) from A. Dickson's laboratory.
Seawater samples for DOC were collected in two pre-combusted (4 h at 450 ∘C) glass ampoules filled with water filtered using a glass syringe filtration system (SGETM) with two pre-combusted 25 mm WhatmanTM GF/F filters. Samples were then acidified with ultrapure orthophosphoric acid (H3PO4), sealed, and stored in the dark at room temperature for later analysis by high-temperature catalytic oxidation (HTCO) (Sugimura and Suzuki, 1988; Cauwet, 1994) on a Shimadzu TOC-L analyzer. Typical analytical precision was ± 0.1–0.5 µM C (SD). Consensus reference materials (http://www.rsmas.miami.edu/groups/biogeochem/CRM.html, last access: March 2021) were injected every 12 to 17 samples to ensure stable operating conditions. DOC concentrations are only available for the 2017 sampling because of a sample's pollution in 2019.
Finally, 1 L of unfiltered seawater was collected in a borosilicate glass bottle and stored on ice during sampling for later measurement of POC and PON contents. In the lab, particulate matter was collected on pre-combusted (4 h at 450 ∘C) WhatmanTM GF/F filters using a Nalgene® vacuum system. The filters were dried at 60 ∘C in the oven for 24 h and stored in airtight glass vials at 4 ∘C in the dark until analysis on a CHN PerkinElmer 2400.
All glass bottles and vials used were pre-combusted, washed with HCl solutions (10 %), and rinsed using Milli-Q water.
Seawater chemistry data were pooled by sampling area (R1, R2, outer, middle, and inner), and differences were tested using the Kruskal–Wallis test followed by the Conover test of multiple comparisons (Benjamini–Hochberg-adjusted). We focused on the effect of the tidal phases (i.e., falling and rising tide) on the seawater chemical composition in the Bouraké lagoon only by attributing each sample a tidal phase between 0 (high tide) and 6 h (low tide) and between 6 and 12 h (high tide). Multiple linear regression was used to assess the adjusted R2 and significance (p<0.05) of the data from 0 to 6 h (falling tide) and from 6 to 12 h (rising tide) separately. Statistical analyses were performed using either Statistica® or R (version 3.2.4; R Core Team, 2018), the latter using the “FSA”, “stats”, and “Conover.test” packages.
2.3 Benthic community characterization and distribution
The benthic community and bottom substrate of the Bouraké lagoon, referred hereafter as biotic and abiotic descriptors, respectively, were assessed in April 2018. A total of 24 30 m long georeferenced transects (T1–T24) were laid in the lagoon along the terraces' edge at similar depths (i.e., ∼1 m), targeting coral-dominated benthic assemblages. On each transect, a 0.5×0.5 m PVC quadrat was placed every meter, and a picture was taken with a waterproof photo camera (Nikon AW130) parallel to the substrate. We made a general description of the bottom (i.e., the various substrates) and a list of the most common and identifiable sessile species for each transect. For each of the 835 pictures collected, we estimated the cover of abiotic (i.e., mud, sand, rock, rubble, dead corals, and unreadable) and biotic descriptors (i.e., branching, massive and soft corals, sponges, macroalgae, and “others”) with photoQuad software both by automatic multi-scale image segmentation regions and manual grid cell counts when necessary.
We used the photos of quadrats, the many other pictures collected during fieldwork, and laboratory morphological observations on collected samples to produce a non-exhaustive species list of corals, macroalgae, and sponges. Corals were comprehensively sampled throughout the Bouraké lagoon and at the reference reef R2, while dominant macroalgal and sponge species were collected in the Bouraké lagoon alone and only if they were encountered at least three times along a transect, likely leading to an underestimation of their diversity. Coral diversity was assessed through photographic and sampling during time-based open-search swims and scuba dives (Hill and Wilkinson, 2004). Whenever possible, scleractinian corals were identified to species level in situ by photographic sampling only. When identification was doubtful in vivo, or when taxa were characterized by small corallite size (<1 mm in diameter) and required additional morphological examination of the skeleton to confirm identification (e.g., genera Acropora, Montipora, and Porites), a fragment of the colony was collected, tagged, cleaned in sodium hypochlorite overnight, rinsed in freshwater, and dried. The reference collection is housed at the Institut de Recherche pour le Développement (IRD), Nouméa. Microscopic examination of the skeletal features allowed species-level identification following the reference literature (Veron and Wallace, 1984; Wallace, 1999; Veron, 2000). Sponges were identified based on their spicules' morphological characteristics (i.e., shape, length, and width) or using a series of morphological descriptors (e.g., shape, size, color, texture, surface ornamentations, fibers) for species without spicules. In the lab, a subsample of the collected sponges was immediately digested using HNO3, and spicules were measured with an optical stereomicroscope. For species without spicules, hand-cut sections of the choanosome and ectosome were observed under a stereomicroscope. Species were identified using the taxonomical keys of Rützler (1978), Hooper and Van Soest (2002), and Pons et al. (2017). Macroalgae and coral identification was based on morphological and anatomical observations following the dedicated literature and referring to specimens housed at IRD Nouméa.
Abiotic and biotic cover percentages averaged per transect and species richness, calculated as the number of species in the transect, were plotted using non-metric multidimensional scaling (nMDS) based on Bray–Curtis dissimilarities (“vegan” package in R) of square-root-transformed data. Finally, the best number of clusters for the whole Bouraké lagoon was determined using the gap statistic method (“cluster” and “factorextra” packages in R) and used for the hierarchical clustering representation (Ward, 1963). The cluster separation was verified with a two-way ANOSIM (ANalysis Of Similarities). Within each cluster, the benthic community and bottom substrate were averaged between transects, and the dominant biotic and abiotic descriptors were selected and used to define the cluster.
Principal component analysis (PCA) was used to visualize the correlation between environmental parameters and the benthic descriptors of transects inside the Bouraké lagoon. The analysis was performed in R (package “FactorMineR”, version 3.2.4; R Core Team, 2018) using data of biotic descriptors (averaged per transect) and the seawater parameters averaged for each sampling area (i.e., outer, middle, and inner reefs).
3.1 Variability in physical environmental parameters
Tidal phases. The predicted tide in the study area (SHOM data) is close to what we measured at R1 and R2 (Fig. 2). It is semidiurnal with a diurnal inequality (M2=0.44 m, S2=0.16 m) and has a small diurnal component (S1=0.15 m). It varies between 0.4 and 1.7 m, depending on the phase of the moon, with a mean of 1.1 m. The tidal range inside the Bouraké lagoon is lower and varies between 0.4 and 1.2 m, with a mean of 0.9 m. At B1 and B2, the tidal signal has an average lag in the predicted and measured tides at the reference stations of 1.5 h for low tide and up to 45 min for high tide.
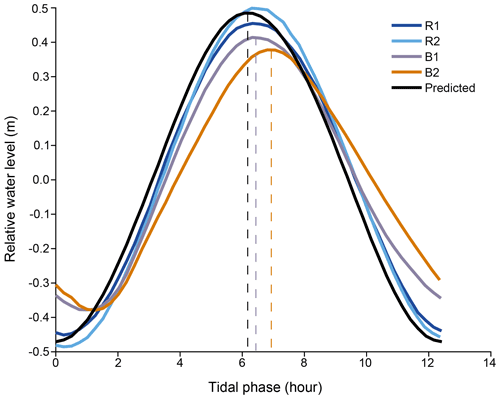
Figure 2Temporal shift between the averaged tides recorded at the reference (Sts R1 and R2) and at the Bouraké (Sts B1 and B2) reefs and the predicted tide calculated for the study area (see SHOM: http://data.shom.fr, last access: February 2021, for the Nouméa harbor).
Temperature. Our results indicate that the water temperature has an annual cycle with lower values during winter (May–October) and higher values in summer (November–April). Daily averaged temperatures at R2, B1, and B2 were not significantly different, while the temperature at R1 was cooler in summer and warmer in winter (Fig. 3a; Table 1) compared to the other stations. On a weekly basis, the averaged diel temperature variation was significantly different between stations: 1.34±0.39 ∘C at R1 and 3.73±0.74 ∘C at B2 (Fig. 3b; Table 1). In a single day, we recorded temperature fluctuations of up to 3.4 ∘C at R1 and 6.5 ∘C at B2.
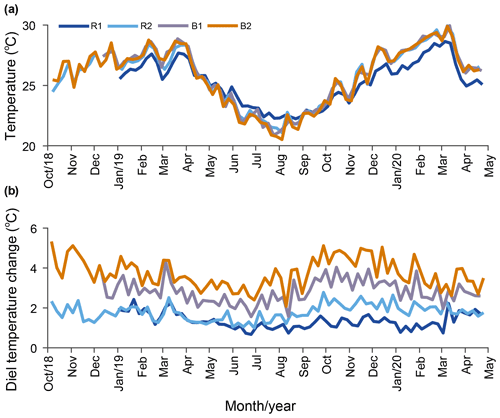
Figure 3Long-term temperature monitoring from October 2018 to April 2020 at the reference (Sts R1 and R2) and Bouraké (Sts B1 and B2) reefs. Data are plotted using weekly averaged temperature (a) and weekly averaged diel (b) changes.
Seawater pH. During the entire study period, pH was measured during 22, 72, 31, and 72 semidiurnal tidal cycles at R1, R2, B1, and B2, respectively. We overlaid all data at a single tidal phase of a 12 h (Fig. 4a) and a 24 h cycle (Fig. 4b).
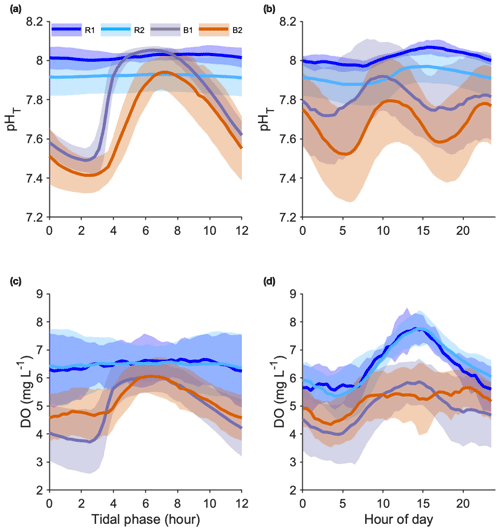
Figure 4Seawater pHT (a, b) and dissolved oxygen (DO) (c, d) variations recorded at the reference (Sts R1 and R2) and Bouraké (Sts B1 and B2) reefs. Data were overlaid at a single tidal phase (12 h) (a, c) and at a 24 h cycle (b, d). Data are 22, 72, 31, and 72 semidiurnal tidal cycles for pH and 36, 79, 34, and 42 semidiurnal tidal cycles for DO, for R1, R2, B1, and B2, respectively.
The pH differed significantly between stations. During the studied period, we recorded means of 8.01±0.04 and 7.89±0.08 pHT units at R1 and R2 and 7.80±0.22 and 7.67±0.23 pHT units at B1 and B2, respectively. At both B1 and B2, pH was strongly correlated with the tidal cycle (Fig. 4a; 82 % and 73 % of the total variance were explained by the tidal harmonic analysis, respectively). In contrast, it was only marginally correlated with the 24 h cycle (Fig. 4b). During each tidal phase, the pH changed on average by about 0.6 units and reached a minimum of 7.23 and a maximum of 8.06 at B2 at low and high tides, respectively (data not shown). The pH oscillations were mainly semidiurnal ( and pHT units for B1 and and pHT units for B2). At stations R1 and R2, pH changed on average by about 0.1 pHT units and was mostly dependent on the 24 h cycle (Fig. 4b; and pHT units for R1 and R2, respectively). Simultaneous short-term pH measurements showed significant spatial differences (Fig. 5; Table 1) between (i) stations A and C, (ii) stations B1 and B2, and (iii) the outer reef in the Bouraké lagoon (St U) and the station outside the system (St T). There were also significant depth-related differences between shallow reefs and bottom water at stations R and S. In both spatial and depth-related analyses, differences were approximately 0.05 to 0.1 pHT units, and we found the lowest values at stations C and B2 and the bottom of the channel (Sts R and S).
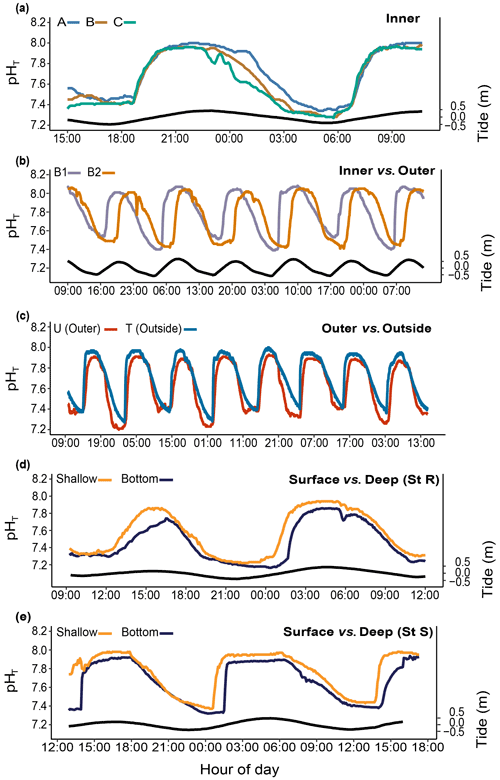
Figure 5Spatial and depth-related short-term changes (24 h) in pHT at stations in the Bouraké lagoon (see Fig. 1 for the station locations). Spatial variations were assessed (a) within the inner reef (Sts A–C); (b) between the inner and the outer reefs (Sts B1 and B2); (c) between the outer reef (but still inside the Bouraké lagoon) and the reef outside the semi-enclosed lagoon (St U vs. T). Depth-related variations were assessed between shallow reefs and the bottom of the channel (d) at the inner reef (St R) and (e) at the outer reef (St S). Tide (black line) refers to St B2.
Dissolved oxygen (DO). DO was measured during 36, 79, 34, and 42 semidiurnal tidal cycles at R1, R2, B1, and B2, respectively. We overlaid all data at a single tidal phase of a 12 h (Fig. 4c) and a 24 h cycle (Fig. 4d). As with pH, the mean diel DO was higher at the reference reefs than in the Bouraké lagoon. During the studied period, mean DO values were 4.89±1.18 and 5.23±0.89 mg L−1 at B1 and B2, respectively, and 6.45±0.95 and 6.48±1.05 mg L−1 at R1 and R2, respectively.
At stations B1 and B2, DO was strongly correlated with the tidal cycle (Fig. 4c; 82 % and 72 % of the total DO variance were explained by the tidal harmonic analysis, respectively) and only marginally with the 24 h cycle (Fig. 4d). DO oscillations were mainly semidiurnal ( mg L−1 and mg L−1 for B1 and B2, respectively) with a substantial diurnal component ( mg L−1 and mg L−1 for B1 and B2, respectively). During a semidiurnal tidal cycle, DO was lower at low tide (Fig. 4c; 3.7 and 4.6 mg L−1 at B1 and B2, respectively) and higher at high tide (Fig. 4c; 6.0 and 6.1 mg L−1 at B1 and B2, respectively). The minimum (1.89 mg L−1) and the maximum (7.24 mg L−1) DO values were both measured at B1 during low and high tides, respectively (data not shown). During a 24 h cycle, DO was lower in the early morning (Fig. 4d; 4.0 and 4.3 mg L−1 at B1 and B2, respectively) and higher in the middle of the day (Fig. 4d; 5.8 and 5.4 mg L−1 at B1 and B2, respectively). In a single day, we recorded DO fluctuations of up to 6.37 mg L−1 at R1 and 4.91 mg L−1 at B2. At stations R1 and R2, DO was mostly dependent on the 24 h cycle ( mg L−1 and mg L−1 for R1 and R2, respectively) with lower values during the night (Fig. 4d; 5.5 mg L−1 at both stations) and higher values in the middle of the day (Fig. 4d; 7.8 mg L−1 at both stations).
Table 1Summary of the statistical analyses applied to the seawater physical and chemical parameters collected between 2016 and 2020 at the Bouraké lagoon (Sts B1, B2, A–R, outer, middle, and inner) and at reference reefs (Sts R1 and R2). Differences in temperature, salinity, and chemical parameters between the Bouraké lagoon and reference reefs and spatial and vertical differences in pH and DO inside the Bouraké lagoon. K–W: Kruskal–Wallis's test; W: Wilcoxon test; GLM: general linear model; D: Dunn's test; T: Tukey's test; C: Conover's test.
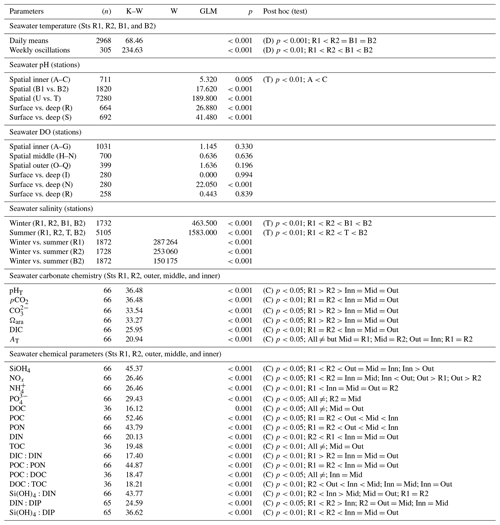
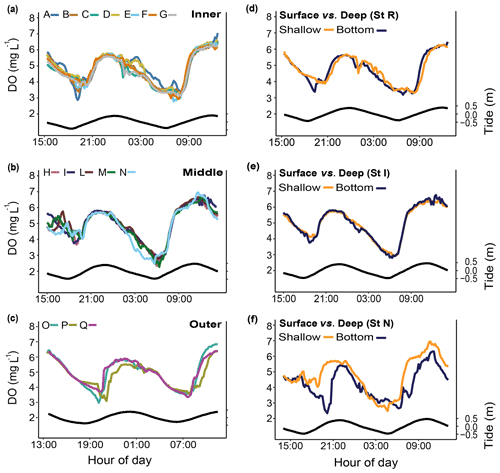
Figure 6Spatial and depth-related short-term measurements (24 h) of dissolved oxygen (DO) at stations in the Bouraké lagoon (see Fig. 1 for the location of the stations). Spatial variations were assessed at (a) the inner reef (Sts A–G), (b) the middle reef (Sts H–N), and (c) the outer reef (Sts O–Q). Depth-related variations between shallow reefs and bottom water within the Bouraké lagoon were assessed (d) at the inner reef (St R) and (e, f) at the middle reef (Sts I and N). Tide (black line) refers to St B2.
Simultaneous short-term DO measurements (Fig. 6; Table 1) did not show significant differences over a 24 h cycle at the inner (Sts A–G; except for some specific deviations, e.g., St A), the middle (Sts H–N), or the outer (Sts O–Q) reef. Dissolved oxygen did not change on a vertical gradient at stations R and I, but the bottom DO was significantly lower at station N.
Salinity (S). Salinity was measured only during two short-term periods in winter 2019 and summer 2020 (Fig. 7).
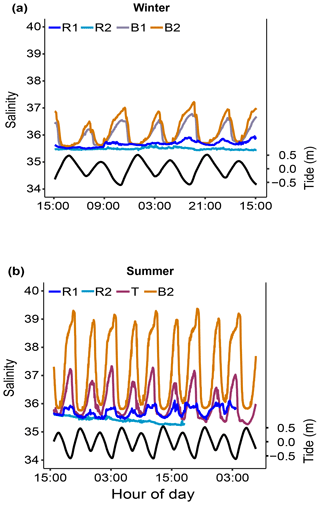
Figure 7Short-term salinity monitoring during (a) the winter of 2019 (from 15 to 18 July 2019) at the reference (Sts R1 and R2) and Bouraké (Sts B1 and B2) reefs and (b) the summer of 2020 (from 29 November to 4 December 2020) at the reference (Sts R1 and R2) and Bouraké (Sts T and B2) reefs. Tide (black line) refers to St B2.
Salinity variations in the Bouraké lagoon were strongly correlated with the tidal cycle, and the highest mean values were measured during both winter and summer. Salinity was lower at reference reefs during both the winter and summer seasons (Fig. 7a and b). The mean salinity during the winter of 2019 was 35.49±0.04 at R1, 35.67±0.10 at R2, 36.00±0.35 at B1, and 36.17±0.47 at B2 with significant differences between stations (Table 1). Salinity in the Bouraké lagoon peaked at 37.22 at B2 during low tide, while it was 35.65 at R1. During the summer of 2020, the mean salinity increased significantly in the Bouraké lagoon, at 37.22±0.53 at B2 reaching 39.37, while it remained lower at R1 at 35.42±0.10 and R2 at 35.71±0.16. During summer 2020, we also measured short-term variations in salinity at station T, which is outside the Bouraké system. Despite its distance from the lagoon entrance, the water mass discharged during falling tide increased seawater salinity also at this station where we measured a maximum value of 37.33 during low tide.
3.2 Diel cycles of carbonate chemistry and chemical parameters
We monitored diel cycles in June 2017 and July 2019 at two reference reefs and three reefs within the semi-enclosed lagoon of Bouraké (i.e., the outer, the middle, and the inner reefs). The seawater carbonate chemistry differed significantly between the reference and the Bouraké lagoon reefs (Figs. 8a–c and S1 in the Supplement; Tables 1 and 2).
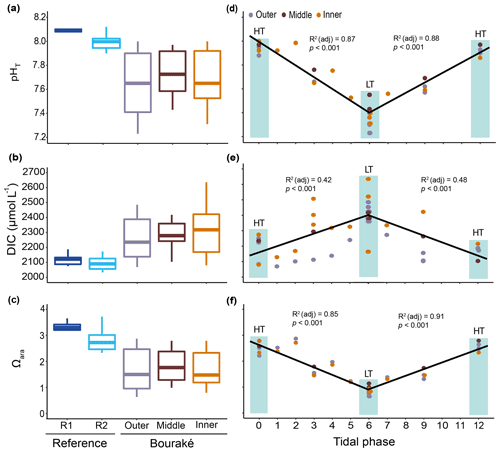
Figure 8Seawater carbonate chemistry measured (pHT) and calculated (DIC and Ωara) during diel cycles in 2017 and 2019 (pooled data; see also Fig. S1) at R1, R2, and the Bouraké reefs (outer, middle, and inner) (a–c). Boxes (n=6–14) represent the interquartile range (25th and 75th percentiles); the horizontal line is the median, and the whiskers represent the data range (i.e., minimum and maximum). Changes are illustrated across a 12 h tidal phase in the Bouraké lagoon (d–f). Linear regression lines are plotted for each falling (HT to LT) and rising (LT to HT) tide. Shaded boxes have only a graphical significance and only suggest the tide changes during measurements.
The reference reefs R1 and R2 had higher pH, , and Ωara and lower DIC and pCO2 than the outer, middle, or inner reefs in the Bouraké lagoon. The range of values in the lagoon was similar to our previous measurements (see above), with levels of pCO2>2000 µatm and levels of Ωara<1. By assigning a tidal phase (from 0 to 12 h) to each sample taken in the Bouraké lagoon (all reefs combined), our diel measurements showed significant regressions, either positive or negative, depending on the seawater carbonate parameter and the tidal phase (Figs. 8d–f and S1; Table S3). For example, the DIC reached the highest value of 2635 µmol L−1 during low tide at the inner reef with an average of 2315.1±168.1 µmol L−1, while reached the lowest value of 50.10 µmol kg−1 during low tide at the inner reef with an average of 109.02±45.59 µmol kg−1.
Most of the chemical parameters were, in general, more concentrated (up to 5 times) in the Bouraké lagoon than at the reference reefs R1 and R2, and they increased from the outer to the inner reef (Figs. 9a–d and S2; Tables 1 and 2).
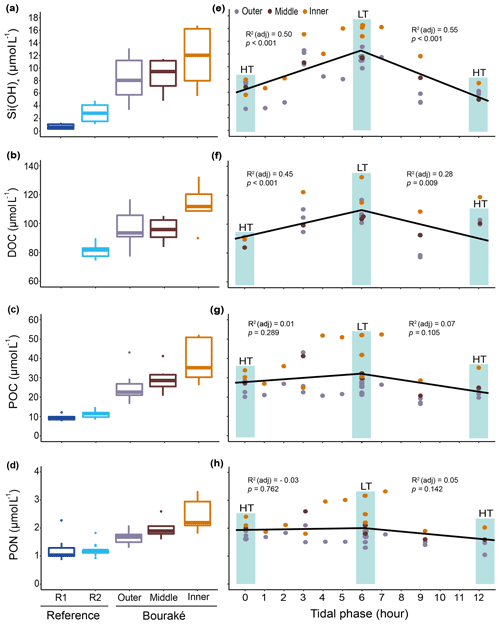
Figure 9Orthosilicic acid, organic carbon, and nitrogen parameters measured during diel cycles in 2017 and 2019 (pooled data; see also Fig. S2) at R1, R2, and the Bouraké reefs (outer, middle, and inner) (a–d). Boxes (n=6–14) represent the interquartile range (25th and 75th percentiles); the horizontal line is the median, and the whiskers represent the data range (i.e., minimum and maximum). Changes are illustrated across a 12 h tidal phase in Bouraké (e–h). Linear regression lines are plotted for each falling (HT to LT) and rising (LT to HT) tide. Shaded boxes have only a graphical significance and only suggest the tide changes during measurements.
In addition, despite the Bouraké lagoon receiving new seawater during the rising tide, only Si(OH)4 concentrations related to the change in tide showed either a positive or negative correlation with the falling and the rising tide (Figs. 9e–h and S2; Table S3). It reached the highest value of 16.74 µmol L−1 during low tide at the inner reef with an average of 11.93±4.27 µmol L−1 and the lowest value of 3.38 µmol L−1 during high tide at the outer reef with an average of 8.35±3.14 µmol L−1, 3 to 6 times higher than at the reference stations R2 and R1. The lack of a clear effect of the tide for the other chemicals causes their accumulation inside the lagoon system. Ratios of organic and inorganic carbon, nitrogen, and nutrients showed significant differences between the Bouraké lagoon and the reference reefs (Tables 1 and 2) with higher average POC:PON, POC:DOC, Si(OH)4:DIN, Si(OH)4:DIP (DIN and DIP signify dissolved inorganic nitrogen and phosphorus), and DIN:DIP () and lower average DIC:DIN and DOC:TOC in the Bouraké lagoon compared to reference reefs.
3.3 Benthic community distribution and species identification
Benthic community distribution. The cluster analysis and nMDS allowed the 24 transects in the Bouraké lagoon to be grouped in six clusters corresponding to habitat descriptors, namely clusters A, B1, B2, C1, C2, and C3 (Fig. 10a; see Fig. S3 for details of the community descriptors and Table S4 for transect averaged data). The nMDS produced an exhaustive representation of the Bouraké lagoon's benthic communities and abiotic features (2-dimensional stress=0.136) confirmed by ANOSIM (global R=0.948, p=0.001). For the benthic community distribution, a clear separation (95 % confidence interval represented by the ellipse in Fig. 10a) was found for cluster A and B2, including most transects located at the middle and inner reefs (Fig. 10b).
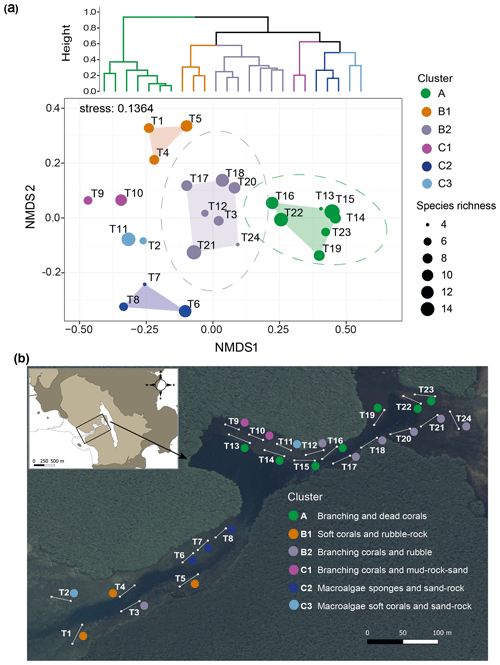
Figure 10Benthic community and bottom substrate characterization in the Bouraké lagoon. Hierarchical Ward's method cluster dendrogram (a) and non-metric multidimensional scaling (nMDS) of abiotic and biotic percent cover and species richness per transect (T1–T24) (a). Colored circles represent clusters of transects identified in the dendrogram; circle size corresponds to species richness. Ellipses represent 95 % confidence intervals. Dominant biotic and abiotic descriptors were used to describe the benthic community and bottom substrate for each cluster in (b). The satellite image is from Georep New Caledonian database (https://georep.nc/), and the QGis software was used for transect georeferencing.
For both, branching corals were the dominant biotic descriptor with an average of 81 % and 31 % coverage, respectively (Fig. S3), with a maximum of 96 % in cluster A (T23; Table S4). Among branching corals, the two most abundant genera were Acropora and Montipora (data not shown). For the abiotic features of the substrate, dead corals and rubble characterized transects in clusters A and B2, respectively. Species richness (Fig. 10a) was high in all transects of the Bouraké lagoon except T13 and T24, both found at the middle and inner reefs' limits. The abiotic substrate of cluster C1 was characterized by 66 % mud, 12 % rocks, and 10 % sand (see Table S4 for detailed cover data per transect). Only a few branching corals (<10 %) were found, but species richness was relatively high (6 at T9 and 10 at T10). Cluster C2 was distinct, which is not surprising due to its location in a relatively shallow convergence zone that divides the lagoon into two parts. There, the substrate is made of coarse sand (13 %) and rocks (12 %) and is mainly colonized by macroalgae and sponges (31 % and 32 %, respectively). Species richness in the area was heterogeneous and ranged from 4 to 12. Dictyota spp. and Halimeda discoidea were the main macroalgal species, while Rhabdastrella globostellata was the dominant sponge species in the area. Cluster B1, located at the outer reef, is characterized by an abundance of soft corals (48 %) and rubble (21 %), as well as high biological richness (Fig. 10a). Cluster C3 is characterized by coarse sand (49 %), rocks (17 %), and a few benthic organisms such as macroalgae (10 %) and soft corals (8 %).
Principal component analysis (PCA) reduced the multicollinearity problem and the first two principal components accounted for a cumulative 71.2 % of the dataset variance. In particular. The PCA plot (Fig. 11) allowed us to assign the transects to three distinct groups depending on their position in the study area.
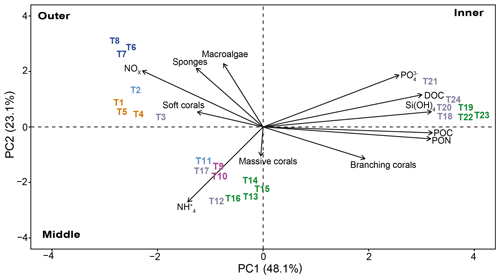
Figure 11Principal component analysis (PCA) between benthic community (macroalgae, sponges, corals, branching corals, and massive corals) data and environmental parameters (PON, POC, DOC, Si(OH)4, , NOx, and ) measured for each transect (n=24). The length of the vectors indicates the contribution of each parameter. The first dimension explains 48.1 % of the variance, and the second dimension explains 23.1 % of the variance. Colors refer to the cluster subdivision (Fig. 10).
The distinction among transect groups in the biplot is clear, and they are grouped based on their location at the outer, middle, or inner lagoon reefs. The outer reef sites are characterized by sponges, macroalgae, and soft corals, which appear to correlate with NOx. Conversely, branching corals are the shared and characteristic feature of the middle and inner reef sites. However, these two reef groups differ in their major correlation to for middle reef sites and POC, DOC, Si(OH)4, and for the inner sites.
Species identification. A total of 66 coral species were identified from the Bouraké lagoon (Table S5). Most of the species belong to the family Acroporidae (17 Acropora species, 2 Anacropora, and 4 Montipora) and Merulinidae (15 species). In total, 58 species were identified from the reference reef R2, 29 of which were also found in the Bouraké lagoon. Overall, the hard coral fauna within the lagoon was characterized by species commonly found around Grande Terre, with some of them typically found in turbid coastal environments such as Pseudosiderastrea tayamai and Heliofungia actiniformis. They were not encountered at the reference site. Remarkably, two New Caledonian endemic coral species, Cantharellus noumeae and Polycyathus fulvus, both described from coastal habitats characterized by terrigenous sediment inputs (Nouméa lagoon and Prony Bay, respectively), were common at the innermost reef of the Bouraké lagoon. The Bouraké lagoon also had 28 species of macroalgae (14 Phaeophyceae, 9 Chlorophyta, and 5 Rhodophyta), including the crustose coralline algae Lithothamnium sp., Lithophyllum sp., and Hydrolithon reinboldii, and 11 sponge species belonging to eight families of the class Demospongiae (Tables S5 and S6), which were found mainly in the coral matrix and sediment.
Marginal and extreme natural environments are increasingly used to predict the future of reefs in a changing world (Camp et al., 2018). In these environments, one or the combination of more environmental conditions differ from present-day values, providing an opportunity to assess the resilience of organisms and to study their adaptive mechanisms in a natural environment. Coral reefs, exposed to seawater pH and temperature values that are close to or even worse than those expected for the future, have likely developed physiological trade-offs and expressed molecular changes that allow them to survive suboptimal and extreme conditions (Kurihara et al., 2021). When using these natural laboratories to predict species responses to future environmental conditions, it is essential to take a multi-scale approach that incorporates the spatial and temporal variability in the key physical and chemical parameters characterizing the study site (e.g., Vizzini et al., 2013; Camp et al., 2018; Aiuppa et al., 2021). Here, we mapped the spatial and temporal variability in the physical and chemical parameters in the semi-enclosed lagoon of Bouraké, which is likely one of the most suitable natural laboratories to study the adaptation of corals to the combination of acidification, warming, and deoxygenation (Camp et al., 2017). There, we found an unprecedented number of benthic species, including two New Caledonian endemics, thriving under chronic suboptimal conditions that fluctuate with the tide. While the exact mechanisms explaining their resilience remains to be discovered, our study provides a compelling basis and fundamental baseline for using this site as a natural laboratory to investigate species' responses to a combination of stresses in their natural environment.
4.1 Physical and chemical characteristics of the Bouraké lagoon
The Bouraké lagoon covers an estimated area of ca. 20 ha that penetrates a mangrove forest, which is large enough to assess the combined effects of extreme environmental conditions at an established coral reef ecosystem. Our multi-scale approach confirmed previous findings (Camp et al., 2017), showing that the Bouraké lagoon is hottest (ranging from 17.5–33.8 ∘C), more deoxygenated (ranging from 1.87–7.24 mg L−1), and more acidic (ranging from 7.23–8.10 pHT units) when compared to neighboring reefs. Besides, we found that salinity was significantly higher than at the reference reefs during both winter and summer (with maxima of 37.22 and 39.37, respectively).
We found several marked differences in the environmental conditions between the Bouraké lagoon and the reference reefs, both in the absolute range and in the variability in the measured environmental parameters.
First, the seawater temperature is higher in summer in the Bouraké lagoon (Fig. 3), but it is also colder during winter, resulting in an annual temperature range of 17.5–33.8 ∘C. We compared temperatures recorded at Bouraké lagoon to those of the reference St R2, which showed the most typical temperature range for shallow water temperatures in the south of New Caledonia (i.e., 22–28 ∘C; Varillon et al., 2021). We notice that in Bouraké, temperatures were 40 % of the time above 28 ∘C during the summer of 2020, while winter temperatures were on average 46.5 % of the time lower than 22 ∘C. While warming is considered the main threat for coral reefs, low temperatures (<20 ∘C) can cause coral bleaching by inducing responses similar to high temperatures, including a reduction in the Symbiodiniaceae cell density and chlorophyll a content (e.g., Saxby et al., 2003; Hoegh-Guldberg and Fine, 2004; Hoegh-Guldberg et al., 2005; Kemp et al., 2011; Bellworthy and Fine, 2021). The negative effect of cold temperatures is even more substantial during neap tides when colonies on the reef crest are exposed to air for hours at low temperatures during cold winters. For example, Porter et al. (1982) and Davis (1982) reported >90 % coral mortality in shallow (<2 m) reefs of the Dry Tortugas following the winter of 1976–1977 when temperatures reached 14 ∘C. This is consistent with our observations (data not shown) during the cold winter of 2019 when we found that the upper 12–20 cm of several massive and branching corals had died.
Second, in the Bouraké lagoon, benthic assemblages are continuously exposed to large fluctuations in the main environmental parameters toward suboptimal values. Some of these environmental fluctuations are entirely predictable. For instance, marine organisms are exposed to a temperature fluctuation of about twice the reference reefs' amplitude (up to 6.5 vs. 3.5 ∘C) in a single day. Dissolved oxygen fluctuations were similar between stations but in a significantly different range: 3.7 to 6.8 mg L−1 at B2 and 5.4 to 7.8 mg L−1 at the reference stations. According to the organisms tolerance to DO fluctuation, which is quite unknown in corals, low DO concentrations can change fish tidal migration in the mangrove (Dubuc et al., 2019). Besides, our tidal modeling revealed that, at the reference reefs, pH, DO (Fig. 4), and temperatures (data not shown) slightly increased in the afternoon and decreased during the night. This finding agrees with what should be expected from reef metabolic activities and daily cycles, but, in the Bouraké lagoon, these parameters, including salinity (Fig. 7), are entirely driven by tides. Here, seawater pH and DO varied between extremely low values at low tide and close-to-normal values during high tide (see also Fig. 8a–c for pH). Finally, we found that the timing of the tide was out of phase between sites, with a delay of about 45 min at high tide and 1.5 h at low tide in the Bouraké lagoon (Fig. 2).
The unique environmental conditions measured in the Bouraké lagoon are linked to its unique topographical and geomorphological characteristics, the resulting water circulation, and the direction of the tide. New water from the lagoon enters through the channel at each rising tide and flows into the semi-enclosed lagoon towards the large mangrove area behind it. This water initially had ambient values of pH, temperature, and dissolved oxygen, but, during the trip, it mixes with the acidic, warm, and deoxygenated water in the system and the mangrove area, therefore gradually changing from its original values. Inside the mangrove forest, we hypothesize that the water chemistry further changes due to the metabolic reactions in the sediments and mangrove roots (e.g., Alongi et al., 2004; Bouillon et al., 2007; Gleeson et al., 2013; Call et al., 2015). Conversely, for a falling tide, the seawater becomes gradually more acidic, hot, and oxygen-depleted because the water that resided in the mangrove area gradually drains out of the system. This takes about 6 h, during which the vast reservoir of shallow mangrove water continues to be chemically altered, becoming increasingly acidic, oxygen-depleted, and hot. As a result, we measured significant spatial differences in pH between the outer reef (the entry of the lagoon) and the inner reef (near the mangrove forest), as well as a considerable delay in the synchronization of the tidal shift (Fig. 5b). Interestingly, because the volume of seawater discharged in 6 h is so large, it affects also the area outside the system where we measured similar seawater conditions as inside, even if it mixes with the main lagoon's water (see Fig. 5c, St U vs. St T). It means that the area (and the organisms) affected by the suboptimal parameters is larger than previously thought. The species living in this area have likely developed specific mechanisms to withstand the drastically fluctuating environmental conditions, and as such, they warrant further attention.
Since the fluctuations are linked to tidal phases, it could be argued that organisms living in the Bouraké lagoon may benefit from periods of normal conditions at high tide, during which they can recover from the stress they have experienced at low tide (e.g., Rivest et al., 2017). While this could be partially the case for species living on the outer reef, close to the main lagoon, the environmental conditions inside the Bouraké lagoon rarely reach normal values (Fig. 8a–c) and persist longer as the low tide is delayed by 1.5 h compared to the reference reef (Fig. 2).
Preliminary results from a hydrodynamic model of the study site suggest that tide-associated water mass movements are spatially heterogeneous and likely to play an essential role in shaping coral resilience to extreme conditions (see discussion below). Indeed, one can imagine a single water mass moving with the same physical characteristics from the mangrove area towards the outer reefs or in the opposite direction depending on the tide. However, the complex geomorphology of the Bouraké lagoon, its bottom topography, and the complex web of coral reefs and mangrove trees on the edges deviate and probably change the seawater's physical and chemical properties. We measured significant spatial differences in pH within each reef area (inner, middle, and outer reefs; Fig. 5a and b), as well as throughout the water column (i.e., between the surface and the bottom; Fig. 5d and e). In general, bottom seawater was 0.1–0.2 pHT units lower than the surface probably due to a pumping mechanism by the water mass of more acidic pore water from the sediments. The pH also differed spatially within the inner reef by up to 0.3 pHT units (for instance St A vs. C; Fig. 5a), perhaps due to the influence of stagnant water on the mangrove edges or a more intense metabolic activity by the local benthic community. Dissolved oxygen concentrations between the surface and the bottom were only significantly different at St N at the middle reef (Fig. 6f). This station is mainly characterized by mud as the current is lower than anywhere else in the Bouraké lagoon. Here, the sediment's biological activity possibly traps the oxygen making it less available to the water column.
Table 2Summary of the seawater physical and chemical data (mean±SD) measured in July 2017 and June 2019 and calculated using the CO2SYS package. Data were pooled and averaged per station; nd=not determined. Reference reefs: Sts R1 and R2; Bouraké lagoon stations: outer, middle, and inner.
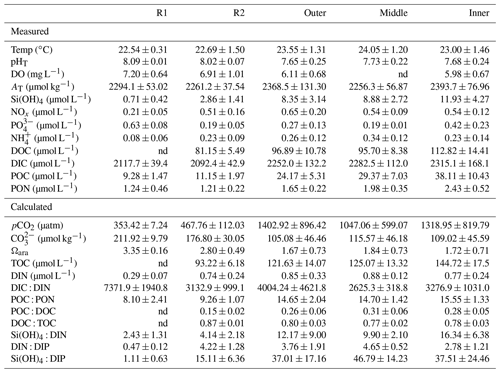
We can assume that, throughout the Bouraké lagoon, organisms are exposed to extreme and fluctuating suboptimal physical parameters, such as pH, and DO which are more pronounced on the bottom and last longer – and with more extreme values – at the inner reefs. It is also true for some of the seawater chemical parameters we measured, which show higher concentrations in the Bouraké lagoon than at the reference reefs (see Table 2). For instance, we found that orthosilicic acid, phosphate, dissolved and particulate organic carbon, and particulate organic nitrogen were 1.4- to 4.2-fold more concentrated at the inner reef than at station R2. Within the Bouraké lagoon in general, chemicals increased from the outer to the inner reef, and except for [NOx], [NH4]+, and [PO4]3−, they never return to “normal” values despite the Bouraké system receiving new seawater during the rising tide. The high concentrations in orthosilicic acid and organic compounds, both dissolved and particulate, are due to the combined effect of more acidic and organic-rich water coming out of the mangrove forest during a falling tide (Fig. 9) and the system's particular morphology, limiting the complete renewal of the seawater, especially at the inner reefs.
4.2 Effects of physical and chemical conditions on species distribution
Extreme environmental conditions, such as those measured in the Bouraké lagoon, are known to strongly affect the metabolism, growth, and even survival of several marine organisms, particularly those tolerating only a narrow range of environmental changes such as scleractinian corals (e.g., Coles and Jokiel, 1977; Hoegh-Guldberg and Smith, 1989; Hoegh-Guldberg, 1999; Fitt et al., 2001). For instance, in situ studies at volcanic CO2 seeps have shown that chronic exposure to ocean acidification (near-future pCO2 levels) can cause a reduction in coral diversity and lower the recruitment and abundances of structurally complex hermatypic corals. Moreover, shifts in competitive interactions between taxa and a decrease in cover and richness of soft corals and sponges were also observed (e.g., Fabricius et al., 2011; Enochs et al., 2015; Sunday et al., 2017; Agostini et al., 2018). However, the Bouraké lagoon features high coral, sponge, macroalgae, and crustose coralline algae (CCA) cover and species richness, adding to the checklist proposed by Camp et al. (2017) an additional 46 coral species (totaling 66 species and 33 genera), 28 species of macroalgae, and 11 species of sponges (Table S5). Such a high number of species has never been reported for mangrove systems characterized by marginal conditions, at least concerning the three major benthic group taxa investigated here (e.g., Yates et al., 2014; Camp et al., 2019). In general, given the extensive literature demonstrating the negative effects of suboptimal abiotic conditions on corals, our findings are unexpected and suggest that corals in the Bouraké lagoon may have developed unique survival and adaptive mechanisms. Some studies have reported similar findings, but none have ever reported such high coral species diversity (e.g., Yates et al., 2014; Schoepf et al., 2015; Shamberger et al., 2018; Camp et al., 2019). For instance, in Palau (Micronesia), the highest coral cover (>60 %) and species diversity (21 scleractinian genera) were found at the lowest pH study site of the Nikko Bay station (Barkley et al., 2015). Studies in the Virgin Islands' mangrove system have reported over 30 coral species growing in marginal conditions (Yates et al., 2014; Rogers, 2017). Similarly, in a recent study of two Australian mangrove lagoons, characterized by relatively extreme environmental conditions (low pH, low oxygen, and high temperature), Camp et al. (2019) identified 12 and 29 scleractinian coral species in the Woody Isles and at Howick Island, respectively. Among the 66 species we identified, 29 of the most abundant coral species in New Caledonia were found both at the reference reef and in the Bouraké lagoon, indicating that species living at our study site are not very different from a typical bay-sheltered fringing reef. There is not an apparent selection in the coral form since massive (e.g., Porites spp.), corymbose (e.g., Pocillopora digitata), phaceloid (Galaxea fascicularis), and branching (Acropora spp.) species are equally abundant, as well as in their thermal and pH tolerance. Further experiments are underway at both the molecular and phenotypic level to better understand the mechanisms of resilience used by the corals from Bouraké.
Remarkably, the two coral species, currently considered New Caledonian endemics, thrive in the innermost benthic assemblages of the Bouraké lagoon, making it not only a unique natural laboratory but a potential conservation priority site. In the Bouraké lagoon, benthos species richness was very high throughout the system, and the distribution of branching and massive corals was spatially heterogeneous (Figs. 10b and S3). Coral cover decreased near the mangrove forest, where the accumulation of fine sediments and exposure to air during low tide do not allow corals' survival (Fig. S3). Branching corals were particularly abundant at the inner and middle reefs, with the highest cover of 96 % at the inner reef (T23; Table S4). They became sparse at the outer reef, even absent at the system's entrance where soft and massive corals dominated. Macroalgae and sponges, including CCA (data not shown), were quite cryptic throughout the Bouraké lagoon but found almost everywhere in the coral matrix and buried in the sediment. They are particularly abundant in a shallow area that divides the lagoon into two parts (transects T6–T8). There, a Venturi effect generates a strong current, and the substrate consists of coarse sand and rocks. The high flow and the bottom characteristics are both likely to promote macroalgae and sponge occurrences (averaged 30 % and 32 % cover, respectively). The sponge Rhabdastrella globostellata is particularly abundant (up to 40 % cover) and forms massive banks embedding adjacent coral colonies. In general, macroalgae and sponge diversity was not particularly rich, with only 28 and 11 species, respectively, likely due to the sampling method. However, in the Indo-Pacific mangrove systems, sponge diversity is, in general, not extremely high, especially if compared to the Caribbean (Nagelkerken et al., 2008). Few studies are available for Indo-Pacific mangrove sponges. The highest diversity was reported in a study in the mangrove forest of Bangka Island (North Sulawesi, Indonesia), where 19 species were found (Calcinai et al., 2017). Our findings on mangrove sponges in such a unique mangrove area in New Caledonia add to the limited knowledge of sponge species diversity in Pacific mangrove systems.
Water flow may play a critical role in the response of organisms to acidification (Cornwall et al., 2014; Hurd, 2015; Comeau et al., 2019), warming (Schoepf et al., 2018), and deoxygenation (Hughes et al., 2020). It has been reported to affect the diffusion boundary layer (i.e., a thin layer of stagnant water located around aquatic organisms) of corals, CCA, and other calcareous macroalgae, altering their ability to calcify at low pH and to excrete metabolic wastes (reviewed in Nelson and Altieri, 2019). Knowing that flow speed could play a critical role which deserves further investigations and measurements at the study site, we found two hypotheses to explain the high diversity of the Bouraké species and their resilience to suboptimal parameters: (i) the species may benefit from the constant fluctuations of the physical and chemical parameters, and (ii) the species may benefit from heterotrophic inputs of the mangrove (in term of nutrients and organic matter).
Regarding environmental fluctuations, we measured averaged daily variations in temperature, DO, and pH of about 3.79 ∘C, 3.80 mg L−1, and 0.63 pHT units, respectively. The temperature fluctuated by up to 6.55 ∘C in a single day. Frequent exposure to stressful temperatures can induce acclimatization or adaptation in corals if the period of exposure is short enough to avoid mortality (Oliver and Palumbi, 2011; Palumbi et al., 2014; Schoepf et al., 2015; Rivest et al., 2017; Safaie et al., 2018). For example, in the Kimberley region (Australia), corals regularly exposed to temperatures up to 37 ∘C, with daily variations of up to 7 ∘C, appear less prone to bleaching and grow and calcify at rates comparable to corals in more thermally stable environments (Dandan et al., 2015; Schoepf et al., 2015, 2020). However, they are not immune to extreme heat stress events (Le Nohaïc et al., 2017). Another example is the corals at the back reef and shallow pools around the island of Ofu (American Samoa), which despite occasional daily fluctuations of up to 6 ∘C and an average daily temperature of 29 ∘C in summer (Piniak and Brown, 2009; Oliver and Palumbi, 2011) sustain reasonable levels of coral cover (25 %–26 %) and high diversity (Craig et al., 2001). Some studies tend to indicate that corals depended on the a priori “frontloading” of genes involved in heat resistance in the coral host and/or the host's ability to adjust its physiology during short-term (weeks) environmental changes (Barshis et al., 2013; Palumbi et al., 2014). However, physiological adjustments have an energetic cost that corals cannot sustain in the long term, affecting other metabolic functions such as calcification. It could explain in part the lower calcification rates observed in some coral species, as seems to be the case for corals in the Bouraké lagoon (e.g., Camp et al., 2017). There is also evidence that exposure to short-term oscillations in low pH, alone or in combination with high temperature, can mitigate the impact of extreme environmental stressors on corals (Warner et al., 1996; Oliver and Palumbi, 2011; Dufault et al., 2012; Schoepf et al., 2015; Safaie et al., 2018). Species in the Bouraké lagoon appear to be thriving despite the large seawater pH variability (up to 0.6 pHT units). In general, species exposed to pCO2 fluctuations above 500 µatm in their natural environment display enhanced plasticity to future ocean acidification scenarios (Vargas et al., 2017; Carstensen and Duarte, 2019). For example, fluctuations in pH could increase CCA tolerance to ocean acidification by providing respite periods at ambient pH (Rivest et al., 2017), during which organisms can calcify at a standard rate, compensating for decreased calcification during periods of lower pH (Comeau et al., 2013; Cornwall et al., 2018). Diurnal variability in pH, typical of more variable pH habitats, may confer tolerance to resident calcifying species via the selection of individuals better adapted to survive in these environments (Rivest et al., 2017; Kapsenberg and Cyronak, 2019), although trans-generational acclimatization is required (Cornwall et al., 2020).
In addition to changes in temperature and pH, which are the two most commonly tested environmental parameters, oxygen must also be considered. Despite the impact of reduced oxygen levels on and natural fluctuations in coral reefs having yet to be established, low DO (2–4 mg L−1) seems to increase the sensitivity of branching corals, resulting in a decline in coral health, bleaching, and tissue loss (Haas et al., 2014; Hughes et al., 2020; Alderdice et al., 2021). Our study shows that although the Bouraké system can reach conditions close to hypoxia for several coral species (<3 mg L−1; Fig. 4), these latter seem to have promoted compensation mechanisms that allow them to survive in these conditions. The natural laboratory of Bouraké, where DO fluctuates with the tide, in combination with other environmental stressors, offers a perfect setting to test the practically unknown effects of deoxygenation in reef-building corals exposed to acid and hot conditions (Nelson and Altieri, 2019; Hughes et al., 2020).
Although corals appear to possess cellular mechanisms to counteract short-term osmotic changes (Mayfield and Gates, 2007), high and fluctuating salinity is a possible additional stress that corals living in the Bouraké lagoon have to face daily, adding up to the already long list of suboptimal environmental parameters. In situ studies have demonstrated that acute and prolonged decrease in salinity can affect the coral photosynthetic efficiency, resulting in a reduction of the amount of energy transferred to corals (Muthiga and Szmant, 1987; Manzello and Lirman, 2003), and induce coral death (e.g., Jokiel et al., 1993). The response of corals to a change in salinity is related to the strength and duration of the hypo-hypersaline exposure and the species tolerance. For example, Stylophora pistillata seems able to acclimate more effectively to hypo- rather than to hyper-saline conditions (Ferrier-Pagès et al., 1999). Further experiments are needed to assess the effect of high and fluctuating salinity on the physiology of corals in Bouraké.
Besides the hypothesis that environmental variability improves the metabolism of organisms, particularly their resilience to extreme conditions, a series of other physical (e.g., current flow) and chemical parameters (e.g., organic matter) in the Bouraké lagoon may work in combination to offset or enhance these effects. Mangrove habitats are highly productive ecosystems and are sites of intense carbon processing, with a high potential impact on the global carbon budget (e.g., Borges et al., 2003; Dittmar et al., 2006; Bouillon et al., 2014). In the Bouraké lagoon, benthic communities might have access to a range of heterotrophic inputs, nutrients, carbon, and nitrogen sources. These sources can be metabolized by the species to increase their energy budget and cope with the suboptimal parameters, but they can also become toxic if too concentrated or depleted, leading to functional limitations. We measured exceptionally high concentrations of organic and inorganic carbon and nitrogen but also of some nutrients, notably silicates and phosphorus, and we confirmed the potential contribution of the mangrove in those inputs, especially during the falling tide (Figs. 8, 9, S1, and S2; Table 2). We found that dissolved organic carbon contributes significantly to the TOC pool (POC+DOC), with a concentration increase between the reference sites and the Bouraké lagoon, reaching maximum values at the inner reef. High organic matter content can increase DOC availability to corals, providing the sustainable energy to withstand extreme environmental conditions (Levas et al., 2015). Some studies showed that the high organic matter of turbid reefs can support elevated coral heterotrophy that can facilitate energy maintenance during periods of stress (Anthony and Fabricius, 2000).
We also found that nutrients could partially explain the distribution of organisms throughout the Bouraké lagoon (Fig. 11). Indeed, NOx concentrations were higher at the outer reef, dominated by sponges, macroalgae, and soft corals, while was high at the middle reef, and POC, DOC, Si(OH)4, and were higher at the inner reef. Both the middle and inner reefs are characterized by the highest branching coral cover. Nitrates can accumulate inside the host cells, possibly favoring sponges and macroalgae, while ammonium is the preferred source used by coral symbionts (Raven et al., 1992). However, the nutrient negative or positive effect on corals' physiology is difficult to demonstrate experimentally (Atkinson et al., 1995; Szmant, 2002; Bongiorni et al., 2003). Van De Waal et al. (2009) have shown that the performance of organisms depends on concentrations of, and ratios between, different nutrients, as well as between organic and inorganic matter/components and the possible imbalance due to environmental changes. We found that the Bouraké lagoon is mostly N-limited ( and ), which confirms the findings of Justić et al. (1995) and is similar to the conditions observed in other New Caledonian mangroves (Jacquet et al., 2006). Nutrient limitation has been demonstrated to lower the temperature effect at which coral bleaching occurs (Wiedenmann et al., 2013; Ezzat et al., 2016a, 2019), which contrasts with the resilience of Bouraké corals to the warming in the summer of 2016 (10 %–20 % bleaching only) compared to other reefs in New Caledonia (up to 90 % bleaching) (Benzoni et al., 2017). Coral symbionts recycle their host's metabolic wastes and take up dissolved inorganic nitrogen (DIN) and phosphorus (DIP) from seawater (Grover et al., 2003; Pernice et al., 2012; Rosset et al., 2015), both of which are used to produce vital organic molecules. Nutrient starvation can occur when the availability of one type of essential nutrient decreases, resulting in an imbalanced N:P ratio of inorganic nutrients in seawater (Wiedenmann et al., 2013; D'Angelo and Wiedenmann, 2014). Based on our measurements, the N:P ratio in the Bouraké lagoon was 2.8:1 and 4.6:1 at the middle and the reference reefs, respectively, which is lower than the range calculated for average reef waters of 4.3:1 to 7.2:1 (Crossland et al., 1984; Furnas et al., 1995). Although these data should be considered cautiously, they suggest that the Bouraké lagoon seawater is not limited in phosphorus, an essential nutrient in coral resilience to bleaching (Ezzat et al., 2016b; Rosset et al., 2017).
Seawater in the Bouraké lagoon was not limited in Si ( and ; Justić et al., 1995), and Si was 6 times more abundant at the inner reef than elsewhere in the system (Fig. 9; Table 2). Silicates are an important source of nutrition and skeletal construction for primary producers such as sponges. Orthosilicic acid is a biologically available form of silicon that is poorly soluble at a very low pH. In the Bouraké lagoon, pH fluctuates with the tide, and one can expect that orthosilicic acid would rise during the falling tide. This elevated concentration of orthosilicic acid could be involved in the growth and persistence of the Bouraké large banks of Rhabdastrella globostellata, as previously reported for other sponges in the shallow hydrothermal vents in the Pacific Ocean (Maldonado et al., 1999; Cárdenas and Rapp, 2013; Bertolino et al., 2017).
We are still missing information about light, turbidity, current, bacteria biomass, phytoplankton sources, and other biological communities to fully understand this complex and dynamic system and the functioning of this complex and dynamic coral ecosystem. However, our data already demonstrate the Bouraké lagoon's uniqueness as a natural laboratory for studying the adaptive responses of corals and other reef species to the combination of multiple suboptimal environmental parameters, which are, to some extent, worse than those projected for the future. Our investigations indicate that the geomorphology of this unique site has not changed for the last 80 to 100 years and certainly longer. With this in mind, we assume that, at least in the previous century, the environmental conditions remained unchanged, and the corals of the Bouraké lagoon have experienced the current extreme conditions for several generations. Most importantly, the species found in the Bouraké lagoon are not unique to the mangrove habitat. They are common throughout New Caledonia and the southwest Pacific region, suggesting that they have used specific strategies to cope with the suboptimal environmental conditions.
We are also unsure how the different parameters will fluctuate at bay-sheltered reefs over time and under future climate change conditions and if the variability measured in the Bouraké lagoon is representative of the natural fluctuations expected for coastal habitats in the future. Indeed, earth system model simulations, which are mainly based on open-ocean-system models, project that the seasonal amplitude of pH and pCO2 will increase by 81 % in the future climate (Kwiatkowski and Orr, 2018). Projections also indicate that ocean acidity extremes will be more frequent (Burger et al., 2020), which could mean that future diel pH variability will increase even more at sites with the most significant variability today. The situation could be similar for seawater temperature, a parameter that is already high in Bouraké and likely close to the coral's thermal tolerance threshold. If this were to happen in the following decades, the coral reef of Bouraké would face incredibly harsh environmental conditions since physical and biogeochemical parameters measured during this study already exceed future climatic simulations.
We used a multi-scale approach to characterize the physical and chemical environmental parameters of one of the most realistic natural laboratories for extreme environmental conditions, the semi-enclosed lagoon of Bouraké (New Caledonia), and accurately map its benthic community for the first time. We studied several physical and chemical parameters such as pH, dissolved oxygen, temperature, and salinity but also nutrients and organic matter, and we found that (i) they fluctuate between low and high tides, ranging from suboptimal extreme to near normal values, (ii) although predictable according to the tide, they differed spatially, and (iii) suboptimal values persisted longer and were more acute at the inner reef. Our data clearly show that fluctuations are (i) predictable, at least for some of the physical parameters for which we have enough data (i.e., pH, DO, temperature, and salinity), and (ii) mainly driven by the tide, and (iii) seawater nutrient imbalance and organic inputs increase during the falling tide and originate from the mangrove forest and associated sediments. Although several studies suggest that ocean acidification, warming, and, to some extent, deoxygenation will lead to a reduction in biodiversity and increase in bleaching and reef dissolution, in the Bouraké lagoon, we found healthy reef with high coral cover and species richness but also sponges and macroalgae (including CCA). It was beyond the scope of this study to assess the contribution of environmental variability and nutrient imbalance to the organism' stress tolerance under extreme conditions. However, both coexist in the Bouraké lagoon, and we believe there is evidence of their contribution to the survival of organisms in extreme environmental conditions. Our study provides evidence that this is possible in existing natural habitats, giving a glimmer of hope for the future of coral reefs. Further experiments are needed to reveal the mechanisms involved in the organisms' resilience to such conditions. Finally, we provide the compelling basis for using this site as a natural laboratory to study better the multitude of complex stressors acting together on lifelong adapted coral reefs.
The code used in this study is available upon request.
The dataset compiled and analyzed in this study is available on PANGAEA at https://issues.pangaea.de/browse/PDI-29562 (last access: 13 September 2021, login required).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-5117-2021-supplement.
RRM conceived and designed the project with input from FM for the benthic community distribution study. FM and RRM collected the data. FB, CP, CC and BC identified the corals, macroalgae and sponges, respectively. MPP performed all the chemical analyses. FM conducted the data analysis with the help of JA and RRM. FM drafted the manuscript in collaboration with RRM. All co-authors read and edited the final version of the manuscript.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We wish to express our thanks to captains of the IRD vessels and the diving technical staff for their fieldwork assistance. We thank the LAMA laboratory (IRD, Nouméa) and LOMIC laboratory (Banyuls sur mer, France) for the analysis and use of their instruments and facilities. We also thank “Province Sud” for sample collection permits (no. 3413-2019) and Greg and Esme (Chez Esme) for their support and hospitality during field missions.
This study was supported by the French grant scheme Fonds Pacifiques (no. 1976; project SuperCoraux), and data were collected during the SuperNatural 1 and 2 cruises on board R/V Alis (Flotte Océanographique Francaise https://doi.org/10.17600/17003400). Funds for that were awarded to Riccardo Rodolfo-Metalpa. Federica Maggioni received a PhD fellowship (project REEF-ENGINE) from the University of New Caledonia.
This paper was edited by Peter Landschützer and reviewed by two anonymous referees.
Agostini, S., Harvey, B. P., Wada, S., Kon, K., Milazzo, M., Inaba, K., and Hall-Spencer, J. M.: Ocean acidification drives community shifts towards simplified non-calcified habitats in a subtropical-temperate transition zone, Sci. Rep., 8, 11354, https://doi.org/10.1038/s41598-018-29251-7, 2018.
Aiuppa, A., Hall-Spencer, J. M., Milazzo, M., Turco, G., Caliro, S., and Di Napoli, R.: Volcanic CO2 seep geochemistry and use in understanding ocean acidification, Biogeochemistry, 152, 93–115, https://doi.org/10.1007/s10533-020-00737-9, 2021.
Alderdice, R., Suggett, D. J., Cárdenas, A., Hughes, D. J., Kühl, M., Pernice, M., and Voolstra, C. R.: Divergent expression of hypoxia response systems under deoxygenation in reef-forming corals aligns with bleaching susceptibility, Glob. Change Biol., 27, 312–326, https://doi.org/10.1111/gcb.15436, 2021.
Alongi, D. M., Sasekumar, A., Chong, V. C., Pfitzner, J., Trott, L. A., Tirendi, F., Dixon, P., and Brunskill, G. J.: Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land-ocean-atmosphere exchange in peninsular Malaysia, Mar. Geol., 208, 383–402, https://doi.org/10.1016/j.margeo.2004.04.016, 2004.
Anthony, K. R. N. and Fabricius, K. E.: Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity, J. Exp. Mar. Biol. Ecol., 252, 221–253, https://doi.org/10.1016/S0022-0981(00)00237-9, 2000.
Atkinson, M. J., Carlson, B., and Crow, G. L.: Coral growth in high-nutrient, low-pH seawater: a case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii, Coral Reefs, 14, 215–223, https://doi.org/10.1007/BF00334344, 1995.
Barkley, H. C., Cohen, A. L., Golbuu, Y., Starczak, V. R., DeCarlo, T. M., and Shamberger, K. E. F.: Changes in coral reef communities across a natural gradient in seawater pH, Science Advances, 1, e1500328, https://doi.org/10.1126/sciadv.1500328, 2015.
Barkley, H. C., Cohen, A. L., McCorkle, D. C., and Golbuu, Y.: Mechanisms and thresholds for pH tolerance in Palau corals, J. Exp. Mar. Biol. Ecol., 489, 7–14, https://doi.org/10.1016/j.jembe.2017.01.003, 2017.
Barshis, D. J., Ladner, J. T., Oliver, T. A., Seneca, F. O., Traylor-Knowles, N., and Palumbi, S. R.: Genomic basis for coral resilience to climate change, P. Natl. Acad. Sci. USA, 110, 1387–1392, https://doi.org/10.1073/pnas.1210224110, 2013.
Bellworthy, J. and Fine, M.: Warming resistant corals from the Gulf of Aqaba live close to their cold-water bleaching threshold, 1843, PeerJ, 9, e11100, https://doi.org/10.7717/peerj.11100, 2021.
Benzoni, F., Houlbrèque, F., André, L. V., and Payri, C.: Plan d'action rapide et adaptatif en cas de blanchissement corallien: Le cas de la Nouvelle-Calédonie, épisode 2016, synthèse, Sciences de la Mer. Biologie Marine, Rapports Scientifiques et Techniques, 6, 90, 2017.
Bertolino, M., Oprandi, A., Santini, C., Castellano, M., Pansini, M., Boyer, M., and Bavestrello, G.: Hydrothermal waters enriched in silica promote the development of a sponge community in North Sulawesi (Indonesia), European Zoological Journal, 84, 128–135, https://doi.org/10.1080/11250003.2016.1278475, 2017.
Bongiorni, L., Shafir, S., Angel, D., and Rinkevich, B.: Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment, Mar. Ecol. Prog. Ser., 253, 137–144, https://doi.org/10.3354/meps253137, 2003.
Borges, A. V., Djenidi, S., Lacroix, G., Théate, J., Delille, B., and Frankignoulle, M.: Atmospheric CO2 flux from mangrove surrounding waters, Geophys. Res. Lett., 30, 1558, https://doi.org/10.1029/2003GL017143, 2003.
Bouillon, S., Middelburg, J. J., Dehairs, F., Borges, A. V., Abril, G., Flindt, M. R., Ulomi, S., and Kristensen, E.: Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania), Biogeosciences, 4, 311–322, https://doi.org/10.5194/bg-4-311-2007, 2007.
Bouillon, S., Yambélé, A., Gillikin, D. P., Teodoru, C., Darchambeau, F., Lambert, T., and Borges, A. V.: Contrasting biogeochemical characteristics of the Oubangui River and tributaries (Congo River basin), Sci. Rep., 4, 5402, https://doi.org/10.1038/srep05402, 2014.
Boyd, P. W., Cornwall, C. E., Davison, A., Doney, S. C., Fourquez, M., Hurd, C. L., Lima, I. D., and McMinn, A.: Biological responses to environmental heterogeneity under future ocean conditions, Glob. Change Biol., 22, 2633–2650, https://doi.org/10.1111/gcb.13287, 2016.
Burger, F. A., John, J. G., and Frölicher, T. L.: Increase in ocean acidity variability and extremes under increasing atmospheric CO2, Biogeosciences, 17, 4633–4662, https://doi.org/10.5194/bg-17-4633-2020, 2020.
Calcinai, B., Bastari, A., Makapedua, D. M., and Cerrano, C.: Mangrove sponges from Bangka Island (North Sulawesi, Indonesia) with the description of a new species, J. Mar. Biol. Assoc. UK, 97, 1417–1422, https://doi.org/10.1017/S0025315416000710, 2017.
Call, M., Maher, D. T., Santos, I. R., Ruiz-Halpern, S., Mangion, P., Sanders, C. J., Erler, D. V., Oakes, J. M., Rosentreter, J., Murray, R., and Eyre, B. D.: Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring-neap-spring timescales in a mangrove creek, Geochim. Cosmochim. Ac., 150, 211–225, https://doi.org/10.1016/j.gca.2014.11.023, 2015.
Camp, E. F., Nitschke, M. R., Rodolfo-Metalpa, R., Houlbreque, F., Gardner, S. G., Smith, D. J., Zampighi, M., and Suggett, D. J.: Reef-building corals thrive within hot-acidified and deoxygenated waters, Sci. Rep., 7, 2434, https://doi.org/10.1038/s41598-017-02383-y, 2017.
Camp, E. F., Schoepf, V., Mumby, P. J., Hardtke, L. A., Rodolfo-Metalpa, R., Smith, D. J., and Suggett, D. J.: The future of coral reefs subject to rapid climate change: Lessons from natural extreme environments, Frontiers in Marine Science, 5, 4, https://doi.org/10.3389/fmars.2018.00004, 2018.
Camp, E. F., Edmondson, J., Doheny, A., Rumney, J., Grima, A. J., Huete, A., and Suggett, D. J.: Mangrove lagoons of the Great Barrier Reef support coral populations persisting under extreme environmental conditions, Mar. Ecol. Prog. Ser., 625, 1–14, https://doi.org/10.3354/meps13073, 2019.
Cárdenas, P. and Rapp, H. T.: Disrupted spiculogenesis in deep-water Geodiidae (Porifera, Demospongiae) growing in shallow waters, Invertebr. Biol., 132, 173–194, https://doi.org/10.1111/ivb.12027, 2013.
Carstensen, J. and Duarte, C. M.: Drivers of pH Variability in Coastal Ecosystems, Environ. Sci. Technol., 53, 4020–4029, https://doi.org/10.1021/acs.est.8b03655, 2019.
Castillo, K. D., Ries, J. B., Weiss, J. M., and Lima, F. P.: Decline of forereef corals in response to recent warming linked to history of thermal exposure, Nat. Clim. Change, 2, 756–760, https://doi.org/10.1038/nclimate1577, 2012.
Cauwet, G.: HTCO method for dissolved organic carbon analysis in seawater: influence of catalyst on blank estimation, Mar. Chem., 47, 55–64, https://doi.org/10.1016/0304-4203(94)90013-2, 1994.
Codiga, D. L.: Unified Tidal Analysis and Prediction Using the UTide Matlab Functions, September, 59, https://doi.org/10.13140/RG.2.1.3761.2008, 2011.
Coles, S. L. and Jokiel, P. L.: Effects of temperature on photosynthesis and respiration in hermatypic corals, Mar. Biol., 43, 209–216, https://doi.org/10.1007/BF00402313, 1977.
Comeau, S., Edmunds, P. J., Spindel, N. B., and Carpenter, R. C.: The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point, Limnol. Oceanogr., 58, 388–398, https://doi.org/10.4319/lo.2013.58.1.0388, 2013.
Comeau, S., Cornwall, C. E., Pupier, C. A., DeCarlo, T. M., Alessi, C., Trehern, R., and McCulloch, M. T.: Flow-driven micro-scale pH variability affects the physiology of corals and coralline algae under ocean acidification, Sci. Rep., 9, 12829, https://doi.org/10.1038/s41598-019-49044-w, 2019.
Cornwall, C. E., Boyd, P. W., McGraw, C. M., Hepburn, C. D., Pilditch, C. A., Morris, J. N., Smith, A. M., and Hurd, C. L.: Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa, PLoS ONE, 9, e97235, https://doi.org/10.1371/journal.pone.0097235, 2014.
Cornwall, C. E., Comeau, S., DeCarlo, T. M., Moore, B., D'Alexis, Q., and McCulloch, M. T.: Resistance of corals and coralline algae to ocean acidification: Physiological control of calcification under natural pH variability, P. Roy. Soc. B-Biol. Sci., 285, 20181168, https://doi.org/10.1098/rspb.2018.1168, 2018.
Cornwall, C. E., Comeau, S., DeCarlo, T. M., Larcombe, E., Moore, B., Giltrow, K., Puerzer, F., D'Alexis, Q., and McCulloch, M. T.: A coralline alga gains tolerance to ocean acidification over multiple generations of exposure, Nat. Clim. Change, 10, 143–146, https://doi.org/10.1038/s41558-019-0681-8, 2020.
Craig, P., Birkeland, C., and Belliveau, S.: High temperatures tolerated by a diverse assemblage of shallow-water corals in American Samoa, Coral Reefs, 20, 185–189, https://doi.org/10.1007/s003380100159, 2001.
Crook, E. D., Cohen, A. L., Rebolledo-Vieyra, M., Hernandez, L., and Paytan, A.: Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification, P. Natl. Acad. Sci. USA, 110, 11044–11049, https://doi.org/10.1073/pnas.1301589110, 2013.
Crossland, C., Hatcher, B., Atkinson, M., and Smith, S.: Dissolved nutrients of a high-latitude coral reef, Houtman Abrolhos Islands, Western Australia, Mar. Ecol. Prog. Ser., 14, 159–163, https://doi.org/10.3354/meps014159, 1984.
Dandan, S. S., Falter, J. L., Lowe, R. J., and McCulloch, M. T.: Resilience of coral calcification to extreme temperature variations in the Kimberley region, northwest Australia, Coral Reefs, 34, 11044–11049, https://doi.org/10.1007/s00338-015-1335-6, 2015.
D'Angelo, C. and Wiedenmann, J.: Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival, Curr. Opin. Env. Sust., 7, 82–93, https://doi.org/10.1016/j.cosust.2013.11.029, 2014.
Davis, G. E.: A century of natural change in coral distribution at the Dry Tortugas: a comparison of reef maps from 1881 and 1976., B. Mar. Sci., 29, 790, https://doi.org/10.1016/0198-0254(82)90301-6, 1982.
DeCarlo, T. M., Harrison, H. B., Gajdzik, L., Alaguarda, D., Rodolfo-Metalpa, R., D'Olivo, J., Liu, G., Patalwala, D., and McCulloch, M. T.: Acclimatization of massive reef-building corals to consecutive heatwaves, P. Roy. Soc. B-Biol. Sci., 286, 20190235, https://doi.org/10.1098/rspb.2019.0235, 2019.
Dittmar, T., Hertkorn, N., Kattner, G., and Lara, R. J.: Mangroves, a major source of dissolved organic carbon to the oceans, Global Biogeochem. Cy., 20, GB1012, https://doi.org/10.1029/2005GB002570, 2006.
Dubuc, A., Baker, R., Marchand, C., Waltham, N. J., and Sheaves, M.: Hypoxia in mangroves: occurrence and impact on valuable tropical fish habitat, Biogeosciences, 16, 3959–3976, https://doi.org/10.5194/bg-16-3959-2019, 2019.
Dufault, A. M., Cumbo, V. R., Fan, T. Y., and Edmunds, P. J.: Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits, P. Roy. Soc. B-Biol. Sci., 279, 2951–2958, https://doi.org/10.1098/rspb.2011.2545, 2012.
Enochs, I. C., Manzello, D. P., Donham, E. M., Kolodziej, G., Okano, R., Johnston, L., Young, C., Iguel, J., Edwards, C. B., Fox, M. D., Valentino, L., Johnson, S., Benavente, D., Clark, S. J., Carlton, R., Burton, T., Eynaud, Y., and Price, N. N.: Shift from coral to macroalgae dominance on a volcanically acidified reef, Nat. Clim. Change, 5, 1083–1088, https://doi.org/10.1038/nclimate2758, 2015.
Ezzat, L., Towle, E., Irisson, J. O., Langdon, C., and Ferrier-Pagès, C.: The relationship between heterotrophic feeding and inorganic nutrient availability in the scleractinian coral T. reniformis under a short-term temperature increase, Limnol. Oceanogr., 61, 89–102, https://doi.org/10.1002/lno.10200, 2016a.
Ezzat, L., Maguer, J. F., Grover, R., and Ferrier-Pagès, C.: Limited phosphorus availability is the Achilles heel of tropical reef corals in a warming ocean, Sci. Rep., 6, 31768, https://doi.org/10.1038/srep31768, 2016b.
Ezzat, L., Maguer, J. F., Grover, R., Rottier, C., Tremblay, P., and Ferrier-Pagès, C.: Nutrient starvation impairs the trophic plasticity of reef-building corals under ocean warming, Funct. Ecol., 33, 643–653, https://doi.org/10.1111/1365-2435.13285, 2019.
Fabricius, K. E., Langdon, C., Uthicke, S., Humphrey, C., Noonan, S., De'ath, G., Okazaki, R., Muehllehner, N., Glas, M. S., and Lough, J. M.: Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations, Nat. Clim. Change, 1, 165–169, https://doi.org/10.1038/nclimate1122, 2011.
Fabricius, K. E., De'ath, G., Noonan, S., and Uthicke, S.: Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities, P. Roy. Soc. B-Biol. Sci., 281, 20132479, https://doi.org/10.1098/rspb.2013.2479, 2013.
Ferrier-Pagès, C., Gattuso, J. P., and Jaubert, J.: Effect of small variations in salinity on the rates of photosynthesis and respiration of the zooxanthellate coral Stylophora pistillata, Mar. Ecol. Prog. Ser., 181, 309–314, https://doi.org/10.3354/meps181309, 1999.
Fitt, W. K., Brown, B. E., Warner, M. E., and Dunne, R. P.: Coral bleaching: Interpretation of thermal tolerance limits and thermal thresholds in tropical corals, Coral Reefs, 20, 51–65, https://doi.org/10.1007/s003380100146, 2001.
Furnas, M. J., Mitchell, A. W., and Skuza, M.: Nitrogen and phosphorus budgets for the central Great Barrier Reef shelf. Great Barrier Reef Marine Park Authority, available at: http://hdl.handle.net/11017/240 (last access: 13 September 2021), 1995.
Gattuso, J. P., Magnan, A., Billé, R., Cheung, W. W. L., Howes, E. L., Joos, F., Allemand, D., Bopp, L., Cooley, S. R., Eakin, C. M., Hoegh-Guldberg, O., Kelly, R. P., Pörtner, H. O., Rogers, A. D., Baxter, J. M., Laffoley, D., Osborn, D., Rankovic, A., Rochette, J., Sumaila, U. R., Treyer, S., and Turley, C.: Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios, Science, 349, aac4722, https://doi.org/10.1126/science.aac4722, 2015.
Gleeson, J., Santos, I. R., Maher, D. T., and Golsby-Smith, L.: Groundwater-surface water exchange in a mangrove tidal creek: Evidence from natural geochemical tracers and implications for nutrient budgets, Mar. Chem., 156, 27–37, https://doi.org/10.1016/j.marchem.2013.02.001, 2013.
Golbuu, Y., Gouezo, M., Kurihara, H., Rehm, L., and Wolanski, E.: Long-term isolation and local adaptation in Palau's Nikko Bay help corals thrive in acidic waters, Coral Reefs, 35, 909–918, https://doi.org/10.1007/s00338-016-1457-5, 2016.
Grover, R., Maguer, J. F., Allemand, D., and Ferrier-Pagès, C.: Nitrate uptake in the scleractinian coral Stylophora pistillata, Limnol. Oceanogr., 48, 2266–2274, https://doi.org/10.4319/lo.2003.48.6.2266, 2003.
Haas, A. F., Smith, J. E., Thompson, M., and Deheyn, D. D.: Effects of reduced dissolved oxygen concentrations on physiology and fluorescence of hermatypic corals and benthic algae, PeerJ, 2, e235, https://doi.org/10.7717/peerj.235, 2014.
Hall-Spencer, J. M., Rodolfo-Metalpa, R., Martin, S., Ransome, E., Fine, M., Turner, S. M., Rowley, S. J., Tedesco, D., and Buia, M. C.: Volcanic carbon dioxide vents show ecosystem effects of ocean acidification, Nature, 454, 96–99, https://doi.org/10.1038/nature07051, 2008.
Hill, J. and Wilkinson, C.: Methods for ecological monitoring of coral reefs, Australian Institute of Marine Science, Townsville, 117, 2004.
Hoegh-Guldberg, O.: Climate change, coral bleaching and the future of the world's coral reefs, Mar. Freshwater Res., 50, 839–866, https://doi.org/10.1071/MF99078, 1999.
Hoegh-Guldberg, O. and Fine, M.: Low temperatures cause coral bleaching, Coral Reefs, 23, 444, https://doi.org/10.1007/s00338-004-0401-2, 2004.
Hoegh-Guldberg, O. and Smith, G. J.: The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana, J. Exp. Mar. Biol. Ecol., 129, 279–303, https://doi.org/10.1016/0022-0981(89)90109-3, 1989.
Hoegh-Guldberg, O., Fine, M., Skirving, W., Johnstone, R., Dove, S., and Strong, A.: Coral bleaching following wintry weather, Limnol. Oceanogr., 50, 265–271, https://doi.org/10.4319/lo.2005.50.1.0265, 2005.
Holmes, R. M., Aminot, A., Kérouel, R., Hooker, B. A., and Peterson, B. J.: A simple and precise method for measuring ammonium in marine and freshwater ecosystems, Can. J. Fish. Aquat. Sci., 56, 1801–1808, https://doi.org/10.1139/f99-128, 1999.
Hooper, J. N. A. and Van Soest, R. W. M.: Systema Porifera: A Guide to the Classification of Sponges, Systema Porifera, Springer, Boston, MA, 1–7, https://doi.org/10.1007/978-1-4615-0747-5_1, 2002.
Hughes, D. J., Alderdice, R., Cooney, C., Kühl, M., Pernice, M., Voolstra, C. R., and Suggett, D. J.: Coral reef survival under accelerating ocean deoxygenation, Nat. Clim. Change, 10, 296–307, https://doi.org/10.1038/s41558-020-0737-9, 2020.
Hughes, T. P., Anderson, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., Baird, A. H., Baum, J. K., Berumen, M. L., Bridge, T. C., Claar, D. C., Eakin, C. M., Gilmour, J. P., Graham, N. A. J., Harrison, H., Hobbs, J. P. A., Hoey, A. S., Hoogenboom, M., Lowe, R. J., McCulloch, M. T., Pandolfi, J. M., Pratchett, M., Schoepf, V., Torda, G., and Wilson, S. K.: Spatial and temporal patterns of mass bleaching of corals in the Anthropocene, Science, 359, 80–83, https://doi.org/10.1126/science.aan8048, 2018.
Hurd, C. L.: Slow-flow habitats as refugia for coastal calcifiers from ocean acidification, J. Phycol., 51, 599–605, https://doi.org/10.1111/jpy.12307, 2015.
Iglesias-Prieto, R., Galindo-Martínez, C. T., Enríquez, S., and Carricart-Ganivet, J. P.: Attributing reductions in coral calcification to the saturation state of aragonite, comments on the effects of persistent natural acidification, P. Natl. Acad. Sci. USA, 111, E300–E301, https://doi.org/10.1073/pnas.1318521111, 2014.
IPCC: Synthesis Report. Contribution of working groups I. II and III to the fifth assessment report of the intergovernmental panel on climate change, 151.10.1017, 2014.
Jacquet, S., Delesalle, B., Torréton, J. P., and Blanchot, J.: Response of phytoplankton communities to increased anthropogenic influences (southwestern lagoon, New Caledonia), Mar. Ecol. Prog. Ser., 320, 65–78, https://doi.org/10.3354/meps320065, 2006.
Jokiel, P. L., Hunter, C. L., Taguchi, S., and Watarai, L.: Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu, Hawaii, Coral Reefs, 12, 177–184, https://doi.org/10.1007/BF00334477, 1993.
Justić, D., Rabalais, N. N., Turner, R. E., and Dortch, Q.: Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences, Estuar. Coast. Shelf S., 40, 339–356, https://doi.org/10.1016/S0272-7714(05)80014-9, 1995.
Kapsenberg, L. and Cyronak, T.: Ocean acidification refugia in variable environments, Glob. Change Biol., 25, 3201–3214, https://doi.org/10.1111/gcb.14730, 2019.
Kemp, D. W., Oakley, C. A., Thornhill, D. J., Newcomb, L. A., Schmidt, G. W., and Fitt, W. K.: Catastrophic mortality on inshore coral reefs of the Florida Keys due to severe low-temperature stress, Glob. Change Biol., 17, 3468–3477, https://doi.org/10.1111/j.1365-2486.2011.02487.x, 2011.
Kerrison, P., Hall-Spencer, J. M., Suggett, D. J., Hepburn, L. J., and Steinke, M.: Assessment of pH variability at a coastal CO2 vent for ocean acidification studies, Estuar. Coast. Shelf S., 94, 129–137, https://doi.org/10.1016/j.ecss.2011.05.025, 2011.
Kroeker, K. J., Micheli, F., Gambi, M. C., and Martz, T. R.: Divergent ecosystem responses within a benthic marine community to ocean acidification, P. Natl. Acad. Sci. USA, 108, 14515–14520, https://doi.org/10.1073/pnas.1107789108, 2011.
Kurihara, H., Suhara, Y., Mimura, I., and Golbuu, Y.: Potential Acclimatization and Adaptive Responses of Adult and Trans-Generation Coral Larvae From a Naturally Acidified Habitat, Frontiers in Marine Science, 7, 581160, https://doi.org/10.3389/fmars.2020.581160, 2020.
Kurihara, H., Watanabe, A., Tsugi, A., Mimura, I., Hongo, C., Kawai, T., Reimer, J. D., Kimoto, K., Gouezo, M., and Golbuu, Y.: Potential local adaptation of corals at acidified and warmed Nikko Bay, Palau, Sci. Rep., 11, 11192, https://doi.org/10.1038/s41598-021-90614-8, 2021.
Kwiatkowski, L. and Orr, J. C.: Diverging seasonal extremes for ocean acidification during the twenty-first centuryr, Nat. Clim. Change, 8, 141–145, https://doi.org/10.1038/s41558-017-0054-0, 2018.
Le Nohaïc, M., Ross, C. L., Cornwall, C. E., Comeau, S., Lowe, R., McCulloch, M. T., and Schoepf, V.: Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia, Sci. Rep., 7, 14999, https://doi.org/10.1038/s41598-017-14794-y, 2017.
Leopold, A., Marchand, C., Deborde, J., and Allenbach, M.: Water Biogeochemistry of a Mangrove-Dominated Estuary Under a Semi-Arid Climate (New Caledonia), Estuar. Coast., 40, 773–791, https://doi.org/10.1007/s12237-016-0179-9, 2017.
Levas, S., Grottoli, A. G., Warner, M. E., Cai, W. J., Bauer, J., Schoepf, V., Baumann, J. H., Matsui, Y., Gearing, C., Melman, T. F., Hoadley, K. D., Pettay, D. T., Hu, X., Li, Q., Xu, H., and Wang, Y.: Organic carbon fluxes mediated by corals at elevated pCO2 and temperature, Mar. Ecol. Prog. Ser., 519, 153–164, https://doi.org/10.3354/meps11072, 2015.
Maldonado, M., Carmona, M. C., Uriz, M. J., and Cruzado, A.: Decline in Mesozoic reef-building sponges explained by silicon limitation, Nature, 401, 785–788, https://doi.org/10.1038/44560, 1999.
Manzello, D. and Lirman, D.: The photosynthetic resilience of Porites furcata to salinity disturbance, Coral Reefs, 22, 537–540, https://doi.org/10.1007/s00338-003-0327-0, 2003.
Manzello, D. P., Kleypas, J. A., Budd, D. A., Eakin, C. M., Glynn, P. W., and Langdon, C.: Poorly cemented coral reefs of the eastern tropical Pacific: Possible insights into reef development in a high-CO2 world, P. Natl. Acad. Sci. USA, 105, 10450–10455, https://doi.org/10.1073/pnas.0712167105, 2008.
Mayfield, A. B. and Gates, R. D.: Osmoregulation in anthozoan-dinoflagellate symbiosis, Comparative Biochemistry and Physiology – A Molecular and Integrative Physiology, 147, 1–10, https://doi.org/10.1016/j.cbpa.2006.12.042, 2007.
Milazzo, M., Rodolfo-Metalpa, R., Chan, V. B. S., Fine, M., Alessi, C., Thiyagarajan, V., Hall-Spencer, J. M., and Chemello, R.: Ocean acidification impairs vermetid reef recruitment, Sci. Rep., 4, 4189, https://doi.org/10.1038/srep04189, 2014.
Muthiga, N. A. and Szmant, A. M.: The effects of salinity stress on the rates of aerobic respiration and photosynthesis in the hermatypic coral Siderastrea siderea, Biol. Bull., 173, 539–551, https://doi.org/10.2307/1541699, 1987.
Nagelkerken, I., Blaber, S. J. M., Bouillon, S., Green, P., Haywood, M., Kirton, L. G., Meynecke, J. O., Pawlik, J., Penrose, H. M., Sasekumar, A., and Somerfield, P. J.: The habitat function of mangroves for terrestrial and marine fauna: A review, Aquat. Bot., 89, 155–185, https://doi.org/10.1016/j.aquabot.2007.12.007, 2008.
Nelson, H. R. and Altieri, A. H.: Oxygen: the universal currency on coral reefs, Coral Reefs, 38, 177–198, https://doi.org/10.1007/s00338-019-01765-0, 2019.
Oliver, T. A. and Palumbi, S. R.: Do fluctuating temperature environments elevate coral thermal tolerance?, Coral Reefs, 30, 429–440, https://doi.org/10.1007/s00338-011-0721-y, 2011.
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N., and Bay, R. A.: Mechanisms of reef coral resistance to future climate change, Science, 344, 895–898, https://doi.org/10.1126/science.1251336, 2014.
Paytan, A., Crook, E. D., Cohen, A. L., Martz, T. R., Takashita, Y., Rebolledo-Vieyra, M., and Hernandez, L.: Reply to Iglesias-Prieto et al.: Combined field and laboratory approaches for the study of coral calcification, P. Natl. Acad. Sci. USA, 111, E302–E303, https://doi.org/10.1073/pnas.1319572111, 2014.
Pernice, M., Meibom, A., Van Den Heuvel, A., Kopp, C., Domart-Coulon, I., Hoegh-Guldberg, O., and Dove, S.: A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis, ISME J., 6, 1314–1324, https://doi.org/10.1038/ismej.2011.196, 2012.
Pichler, T., Biscéré, T., Kinch, J., Zampighi, M., Houlbrèque, F., and Rodolfo-Metalpa, R.: Suitability of the shallow water hydrothermal system at Ambitle Island (Papua New Guinea) to study the effect of high pCO2 on coral reefs, Mar. Pollut. Bull., 138, 148–158, https://doi.org/10.1016/j.marpolbul.2018.11.003, 2019.
Pierrot, D., Lewis, E., and Wallace, D. W. R.: MS Excel program developed for CO2 system calculations, in ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee, 2006.
Piniak, G. A. and Brown, E. K.: Temporal variability in chlorophyll fluorescence of back-reef corals in Ofu, American Samoa, Biol. Bull., 216, 55–67, https://doi.org/10.1086/BBLv216n1p55, 2009.
Pons, L. N., Calcinai, B., and Gates, R. D.: Who's there? – First morphological and DNA barcoding catalogue of the shallow Hawai'ian sponge fauna, PLoS ONE, 12, e0189357, https://doi.org/10.1371/journal.pone.0189357, 2017.
Porter, J. W., Battey, J. F., and Smith, G. J.: Perturbation and change in coral reef communities, P. Natl. Acad. Sci. USA, 1678–1681, https://doi.org/10.1073/pnas.79.5.1678, 1982.
R Core Team: R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, 2018.
Raven, J. A., Wollenweber, B., and Handley, L. L.: A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs, New Phytol., 121, 19–32, https://doi.org/10.1111/j.1469-8137.1992.tb01088.x, 1992.
Rivest, E. B., Comeau, S., and Cornwall, C. E.: The Role of Natural Variability in Shaping the Response of Coral Reef Organisms to Climate Change, Current Climate Change Reports, 3, 271–281, https://doi.org/10.1007/s40641-017-0082-x, 2017.
Rogers, C. S.: A unique coral community in the mangroves of Hurricane Hole, St. John, US Virgin Islands, Diversity, 9, 29, https://doi.org/10.3390/d9030029, 2017.
Rosset, S., D'Angelo, C., and Wiedenmann, J.: Ultrastructural biomarkers in symbiotic algae reflect the availability of dissolved inorganic nutrients and particulate food to the reef coral holobiont, Frontiers in Marine Science, 2, 103, https://doi.org/10.3389/fmars.2015.00103, 2015.
Rosset, S., Wiedenmann, J., Reed, A. J., and D'Angelo, C.: Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates, Mar. Pollut. Bull., 118, 180–187, https://doi.org/10.1016/j.marpolbul.2017.02.044, 2017.
Rützler, K.: Sponges in coral reefs, in: Coral Reefs: Research Methods, edited by: Stoddart, D. R. and Johannes, R. E., Monographs on Oceanographic Methodology 5, UNESCO, Paris, 1978.
Safaie, A., Silbiger, N. J., McClanahan, T. R., Pawlak, G., Barshis, D. J., Hench, J. L., Rogers, J. S., Williams, G. J., and Davis, K. A.: High frequency temperature variability reduces the risk of coral bleaching, Nat. Commun., 9, 1671, https://doi.org/10.1038/s41467-018-04074-2, 2018.
Saxby, T., Dennison, W. C., and Hoegh-Guldberg, O.: Photosynthetic responses of the coral Montipora digitata to cold temperature stress, Mar. Ecol. Prog. Ser., 248, 85–97, https://doi.org/10.3354/meps248085, 2003.
Schoepf, V., Stat, M., Falter, J. L., and McCulloch, M. T.: Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment, Sci. Rep., 5, 17639, https://doi.org/10.1038/srep17639, 2015.
Schoepf, V., Cornwall, C. E., Pfeifer, S. M., Carrion, S. A., Alessi, C., Comeau, S., and McCulloch, M. T.: Impacts of coral bleaching on pH and oxygen gradients across the coral concentration boundary layer: a microsensor study, Coral Reefs, 37, 1169–1180, https://doi.org/10.1007/s00338-018-1726-6, 2018.
Schoepf, V., Jung, M. U., McCulloch, M. T., White, N. E., Stat, M., and Thomas, L.: Thermally Variable, Macrotidal Reef Habitats Promote Rapid Recovery From Mass Coral Bleaching, Frontiers in Marine Science, 7, 245, https://doi.org/10.3389/fmars.2020.00245, 2020.
Shamberger, K. E. F., Lentz, S. J., and Cohen, A. L.: Low and variable ecosystem calcification in a coral reef lagoon under natural acidification, Limnol. Oceanogr., 7, 714–730, https://doi.org/10.1002/lno.10662, 2018.
Soares, M. de O.: Marginal reef paradox: A possible refuge from environmental changes?, Ocean Coast. Manage., 185, 105063, https://doi.org/10.1016/j.ocecoaman.2019.105063, 2020.
Sugimura, Y. and Suzuki, Y.: A high-temperature catalytic oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of a liquid sample, Mar. Chem., 24, 105–131, https://doi.org/10.1016/0304-4203(88)90043-6, 1988.
Sunday, J. M., Fabricius, K. E., Kroeker, K. J., Anderson, K. M., Brown, N. E., Barry, J. P., Connell, S. D., Dupont, S., Gaylord, B., Hall-Spencer, J. M., Klinger, T., Milazzo, M., Munday, P. L., Russell, B. D., Sanford, E., Thiyagarajan, V., Vaughan, M. L. H., Widdicombe, S., and Harley, C. D. G.: Ocean acidification can mediate biodiversity shifts by changing biogenic habitat, Nat. Clim. Change, 7, 81–85, https://doi.org/10.1038/nclimate3161, 2017.
Szmant, A. M.: Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline?, Estuaries, 25, 743–766, https://doi.org/10.1007/BF02804903, 2002.
Van De Waal, D. B., Verspagen, J. M. H., Lürling, M., Van Donk, E., Visser, P. M., and Huisman, J.: The ecological stoichiometry of toxins produced by harmful cyanobacteria: An experimental test of the carbon-nutrient balance hypothesis, Ecol. Lett., 12, 1326–1335, https://doi.org/10.1111/j.1461-0248.2009.01383.x, 2009.
Vargas, C. A., Lagos, N. A., Lardies, M. A., Duarte, C., Manríquez, P. H., Aguilera, V. M., Broitman, B., Widdicombe, S., and Dupont, S.: Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity, Nature Ecology and Evolution, 1, 0084, https://doi.org/10.1038/s41559-017-0084, 2017.
Varillon, D., Fiat, S., Magron, F., Allenbach, M., Hoibian, T., de Ramon N'Yeurt, A., Ganachaud, A., Aucan, J., and Pelletier, B. H. R.: ReefTEMPS: the observation network of the coastal sea waters of the South, West and South-West Pacific, SEA scieNtific Open data Edition (SEANOE), https://doi.org/10.17882/55128, 2021.
Veron, J. E. N.: Corals of the world, Australian Institute of Marine Science, Townsville, 11, 2000.
Veron, J. E. N. and Wallace, C. C.: Scleractinia of Eastern Australia – Part V. Family Acroporidae, Australian Institute of Marine Science, Monograph Series, 6, 1–485, 1984.
Vizzini, S., Di Leonardo, R., Costa, V., Tramati, C. D., Luzzu, F., and Mazzola, A.: Trace element bias in the use of CO2 vents as analogues for low pH environments: Implications for contamination levels in acidified oceans, Estuar. Coast. Shelf S., 134, 19–30, https://doi.org/10.1016/j.ecss.2013.09.015, 2013.
Wall, C. B., Ricci, C. A., Wen, A. D., Ledbetter, B. E., Delania, E., Mydlarz, L. D., Gates, R. D., and Putnam, H. M.: Shifting baselines: Physiological legacies contribute to the response of reef corals to frequent heatwaves, Funct. Ecol., 35, 1366–1378, https://doi.org/10.1111/1365-2435.13795, 2021
Wallace, C. C.: Staghorn corals of the world: a revision of the coral genus Acropora (Scleractinia; Astrocoeniina; Acroporidae) worldwide, with emphasis on morphology, phylogeny and biogeography, Choice Reviews Online, CSIRO, Collingwood, 1999.
Ward, J. H.: Hierarchical Grouping to Optimize an Objective Function, J. Am. Stat. Assoc., 58, 236–244, https://doi.org/10.1080/01621459.1963.10500845, 1963.
Warner, M. E., Fitt, W. K., and Schmidt, G. W.: The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach, Plant Cell Environ., 19, 291–299, https://doi.org/10.1111/j.1365-3040.1996.tb00251.x, 1996.
Wiedenmann, J., D'Angelo, C., Smith, E. G., Hunt, A. N., Legiret, F. E., Postle, A. D., and Achterberg, E. P.: Nutrient enrichment can increase the susceptibility of reef corals to bleaching, Nat. Clim. Change, 3, 160–164 , https://doi.org/10.1038/nclimate1661, 2013.
Wittmann, A. C. and Pörtner, H. O.: Sensitivities of extant animal taxa to ocean acidification, Nat. Clim. Change, 3, 995–1001, https://doi.org/10.1038/nclimate1982, 2013.
Yates, K. K., Rogers, C. S., Herlan, J. J., Brooks, G. R., Smiley, N. A., and Larson, R. A.: Diverse coral communities in mangrove habitats suggest a novel refuge from climate change, Biogeosciences, 11, 4321–4337, https://doi.org/10.5194/bg-11-4321-2014, 2014.





