the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Comment on “Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum” by K. Michaelian and A. Simeonov (2015)
Lars Olof Björn
This is a comment on Michaelian and Simeonov (2015). Michaelian and Simeonov formulate the leading thought in their article: “The driving force behind the origin and evolution of life has been the thermodynamic imperative of increasing the entropy production of the biosphere through increasing the global solar photon dissipation rate”. I shall in the following try to provide some information that might help to clarify whether this is correct.
Already in the first sentence of their abstract Michaelian and Simeonov formulate the leading thought in their article: “The driving force behind the origin and evolution of life has been the thermodynamic imperative of increasing the entropy production of the biosphere through increasing the global solar photon dissipation rate”.
As long as light travels freely in space, there is no change in the entropy associated with it. If it is scattered, entropy is increased. If we consider only the non-absorbed fraction of the light, a surface reflecting light in a diffuse way (i.e., non-specularly) will increase entropy by scattering, not by changing the photon number; there is, in principle, no difference between non-living substances (e.g., pure sand) and organisms in this respect. Thus we can focus on absorption. An absorbing surface on Earth will eventually cause incident radiation to be converted to diffuse radiation of an increased number of less energetic photons, and thus increase entropy more than a reflecting surface (see, e.g., Delgado-Bonal, 2017), although processes such as photosynthesis can cause a considerable time delay in entropy production. Thus, it appears that if Michaelian and Simeonov are correct, one would expect organisms (in particular phototrophic organisms, or the biosphere) to be less reflecting and more absorbing than dead matter. They explicitly state “living systems reduce the albedo of Earth”. Is this correct?
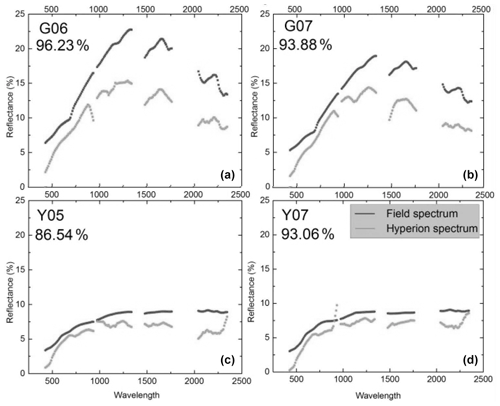
Figure 1Reflectance spectra from the volcano Teide on Tenerife, one of the Canary Islands. The spectra were recorded on the ground (black) or remotely from satellite (gray). The percent values indicate the agreement between the black and gray curves. Top left (G06) is from a site with heavy lichen cover but no plants, and the top right (G07) is heavy lichen cover and some plants. Bottom panels show spectra of bare lava (no vegetation). From Li et al. (2015). Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/, last access: 8 February 2022).
Methanogens represent one of the earliest life-forms on our planet, although not the earliest one (Muñoz-Velasco, 2019), and Ueno et al. (2006) estimate that they existed at least 3.46 Ga (gigayears). Their emission of methane to the atmosphere resulted, under the influence of sunlight, in a colored haze of organic compounds, somewhat similar to that now seen on Titan, the largest moon of Saturn (e.g., Arney et al., 2016). It is likely that a thick haze existed continuously from 3.2 to 2.7 Gyr ago (Domagal-Goldman et al., 2008). Later, “on the eve of the great oxidation event”, 2.7–2.5 Gyr ago, the atmosphere oscillated between a hazy state and periods without thick organic haze (Izon et al., 2015). During the hazy periods no ultraviolet radiation, and only a small fraction of incident visible light, would have penetrated to ground and ocean levels (Arney et al., 2017).
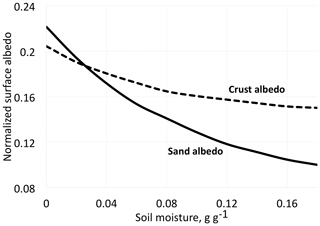
Figure 2Albedo for desert dune sand with and without biological soil crust organisms (BSC) at different moisture contents. Redrawn and simplified from Zang et al. (2013).
The geometric albedo of an astronomical body is the ratio of its total brightness, looking along the direction of illumination, to that of an idealized fully reflecting, diffusively scattering (Lambertian) disk with the same cross section. The geometric spectral albedo of the resulting haze depends on the ratio: the higher it is, the higher the peak wavelength for the reflectance, and the higher the albedo maximum (Arney et al., 2016). For a CO2 pressure of 0.018 bar, at total pressure of 1 bar, and a ratio of 0.21, the haze has a broad reflectance band with a maximum albedo of 0.22 at 750 nm. These values are very sensitive to the ratio. Pavlov et al. (2001) assume a ratio around 1, which can be assumed to have resulted in a thicker haze with a higher reflectance peak at a higher wavelength.
Various other authors date the oldest signs of life as being from 3.22 Ga (Homann, 2019), 3.5 Ga (Djokic et al., 2017; Lepot, 2020), 3.80 Ga (Mojzsis et al., 1996), 3.77 Ga, or possibly 4.28 Ga (Dodd et al., 2017). Hoashi et al. (2009) conclude “These data strongly suggest that oxygenic photoautotrophs flourished in the photic zone of the 3.46 Ga oceans and supplied molecular oxygen to the deep water.” So it is quite possible that other organisms preceded the methanogens. In any case, it is very unlikely that life could have survived prior to 4.4 Ga, because of the formation of the Moon and the “late heavy bombardment” (Bottke and Norman, 2017).
The estimate of the oldest life above, that of Dodd et al., 2017, and that of Djokic et al. (2017) concern life in hydrothermal vent precipitates. There are many other publications suggesting an origin of life in connection with hot springs (see Omran and Pasek, 2020), often at the bottom of the sea (e.g., Martin et al., 2008), out of reach for sunlight. But photosynthesis, and even the complicated oxygenic version, originated very early (see above). These views were recently discussed by Damer and Deamer (2020).
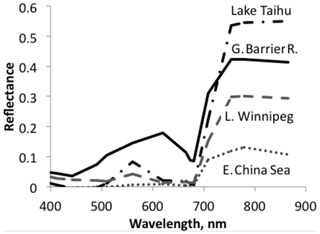
Figure 3A sample of sea and lake reflectance difference spectra redrawn from Qi et al. (2020). The diagram shows reflectance of waters with algae minus adjacent water without algae. These differences are mostly positive, in some cases with the exception for very small negative excursions at short wavelengths.
We must not be restricted to visible light but extend our interest into the infrared, a spectral region to which much of the solar radiation belongs. The quantity we should consider as far as data are available is the hemispherical reflectance, not reflectance in a single direction. Unfortunately, in most cases values of hemispherical reflectance are not available. Directional reflectance spectra (Fig. 1) seem to indicate that vegetated areas may reflect more light throughout the daylight spectrum. The kind of terrestrial life most similar to the newcomers on land may be “biocrusts”. As shown by the example in Fig. 2, these crusts can have albedos exceeding that of the bare ground they inhabit, with an exception only for a very dry state, with less biological activity. There are also cases in which even simple organisms decrease the albedo, one reason being the production of pigments protecting them from sunlight (e.g., Couradeau et al., 2016), a process for which the solar ultraviolet radiation is of particular importance. This fact does not prove that pigments are formed due to a “thermodynamic imperative” for increasing entropy. The presence of moss strongly decreases soil surface albedo, while lichens often have the opposite effect (Blanco-Sacristán, 2019; Xiao and Bowker, 2020).
We shall not go into details of the complications associated with evaporation and other phase transitions; we just point out that forests have a tendency to increase cloudiness and thereby cause increased reflectance by the atmosphere (e.g., Teuling et al., 2017) and thereby counteract entropy increase (while the evaporation itself is associated with an entropy increase).
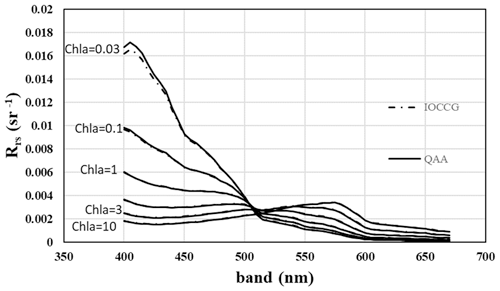
Figure 4Ocean reflectance over 1 radian according to Li et al. (2021) for various concentrations of chlorophyll-a-containing organisms. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/, last access: 6 February 2022).
We can also consider a temporal aspect of this. If sunlight is absorbed by dead matter, it is usually converted to heat and reradiated as heat radiation of ambient temperature within a short time. If it is absorbed by a photosynthetic system, much of the energy is retained for a long time, before eventually being radiated as heat, sometimes after having been processed through several steps in the food chain. Thus, the living system delays entropy increase. Some trees that had collected solar energy sank into swamps more than 250 million years ago, and their reduced carbon was preserved until the present era, when humans started to burn coal and thereby generate entropy. Thus, entropy production was delayed (i.e., the rate of production decreased) thanks to the chlorophyll and the photosynthesis of ancient trees that have been preserved as coal. The production and usage of oil is a similar phenomenon.
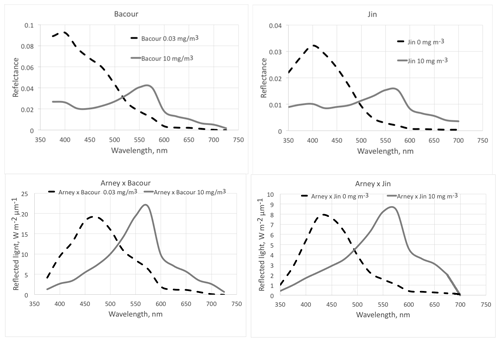
Figure 5The effect of phytoplankton on reflectance of the ocean surface. The dashed lines are for a low chlorophyll content of the surface layer (0 or 0.03 mg m−3) and the solid lines for a high chlorophyll content (10 mg m−3). The top two graphs show the modeled spectra from Bacour et al. (2020) and Jin et al. (2011, diffuse incidence). The geometry is not the same for the two panels; thus the absolute values are not directly comparable. The lower two graphs show the amount of reflected light, assuming an incident Archaean daylight according to the red graph in Fig. 15 (right panel) of Arney et al. (2016).
Much of the Earth surface is covered by water, life probably originated in water, and much of life's evolution has taken place in water. Thus we must also consider the reflectance of water bodies and how their reflectance is affected by life. In addition, aquatic life is easier to deal with, since we do not have to deal with gases (with the exception of dissolved ones, which may escape to the atmosphere, and those forming froth) and processes such as transpiration. Bach et al. (2021) suspect that the increased albedo caused by a recent increase in the Atlantic Ocean of floating Sargassum algae may be more important than the effect of the alga on carbon dioxide and phytoplankton nutrients. A minor effect might arise from increased temperature in the presence of organisms at the surface and probably some increased evaporation (Kahru et al., 1993). However, one should also take into account that the heating that does not take place at the surface if algae are absent there; it takes place deeper down, in a more diffuse way.
Aquatic organisms do not decrease the reflectance of the ocean and other water bodies. This is not always as clear as in the data of Qi et al. (2020), who have published numerous reflection difference spectra for various lakes and ocean surfaces where algae are abundant, i.e., spectra that show the difference in reflectivity for water with and without algae. A sample is reproduced in Fig. 3. In many other cases algae cause a decrease in reflectance at short wavelengths, and an increase above about 500 nm, both for phytoplankton (Jin et al., 2002, 2011; Bacour et al., 2020; Li et al., 2021) (Fig. 5) and for brown and green macroalgae (Qi and Hu, 2021). However, taking the daylight spectrum into account, one finds that the algae increase the total light reflection. The data in Fig. 4 do not extend over a sufficiently wide wavelength range to permit such a comparison, but we show the calculation for Bacour et al. (2020) and Jin et al. (2002) in Fig. 5. Based on the data of both Bacour et al. (2020) and those of Jin et al. (2002), more light is reflected from water containing more algae. This holds on an energy basis, and the increase in reflected light by the algae is even more pronounced when expressed on a photon basis. The light that is not reflected (or emitted as fluorescence) is eventually converted to heat radiation.
Thus the presence of algae increases the reflectivity of oceans and lakes, i.e., counteracts the “degradation” of sunlight to diffuse radiation of longer wavelengths in all directions (not only from the sunlit side of the planet) and the production of entropy.
Aquatic organisms also have indirect effects on the optical properties of both water surfaces and atmosphere. Substances released by algae (e.g., Phaeocystis globosa; Blauw et al., 2010), bacteria (e.g., Rahlff et al., 2021), or other organisms may cause formation of light-reflecting foam on the surface. A much more important effect is the biological increase in cloudiness. In addition to the effect on cloudiness by forest transpiration already mentioned, there is a biological effect on condensation nuclei, which results in increased aerosol and increased cloudiness. Algae form dimethylsulfoniopropionate (DMSP), which decomposes to dimethylsulfide (DMS). In the atmosphere this is converted to various compounds, of which sulfur trioxide and sulfuric acid in particular contribute to aerosol formation and increased reflection of incoming sunlight, thereby decreasing entropy production. I cannot agree with Michaelian and Simeneov (2015) that the following is always true: “Living systems reduce the albedo of Earth and dissipate, through many coupled irreversible processes, shortwave incoming radiation into long-wave radiation, which is eventually returned to space, ensuring an approximate energy balance in the biosphere”.
On the other hand algae on snow decrease the albedo, in one investigation (Lutz et al., 2016) from 0.7 down to, in one case, as low as a 0.5. Yallop et al. (2012) report that under conditions when algae, in addition to photosynthetic pigments, form protective pigments, they decrease Greenland ice albedo from 0.60 (clean ice) to 0.26.
Michaelian and Simeneov (2015) conclude that they have presented evidence that
supports the thermodynamic dissipation theory of the origin of life (Michaelian, 2011), which states that life arose and proliferated to carry out the thermodynamic function of dissipating the entropically most important part of the solar spectrum (the shortest wavelength photons) prevailing at Earth's surface and that this irreversible process began to evolve and couple with other irreversible abiotic processes, such as the water cycle, to become more efficient, to cover ever more completely the electromagnetic spectrum, and to cover ever more of Earth's surface.
I cannot agree that they have presented evidence for this conclusion. The biosphere has certainly evolved and is maintained thanks to a production of entropy associated with the conversion of solar radiation to Earth radiation. For details of this entropy production, I refer to Wu and Liu (2010). They compared and discussed various ways of computing entropy fluxes in the Earth system. There are many examples of how organisms contribute to increased albedo and decreased entropy production.
Ultraviolet radiation has played an important role in the prebiotic synthesis of the molecular species that have made the origin of life possible (Björn, 2015). But several recent publications explore the possibility that the first life emerged in places without any ultraviolet radiation or visible light, such as submarine hydrothermal vents (Altair et al., 2021) or a geyser system driven by nuclear power (Maruyama et al., 2019).
Data are available from the cited literature or from the authors upon request.
The contact author has declared that there are no competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thanks are due to Beth Middleton for comments and language correction of a previous version of the paper and to an anonymous reviewer for helpful comments.
This paper was edited by Carolin Löscher and reviewed by William Martin, Axel Kleidon, and one anonymous referee.
Altair, T., Borges, L. G. F., Galante, D., and Varela, H.: Experimental approaches for testing the hypothesis of the emergence of life at submarine alkaline vents, Life, 11, 777, https://doi.org/10.3390/life11080777, 2021.
Arney, G., Domagal-Goldman, S.-D., Meadows, V. S., Wolf, E. T., Schwieterman, E., Charnay, B., Claire, M., Hébrard, E., and Trainer, M. G.: The pale orange dot: The spectrum and habitability of hazy Archean Earth, Astrobiology, 16, 873–898, https://doi.org/10.1089/ast.2015.1422, 2016.
Arney, G. N., Meadows, V. S., Domagal-Goldman, S. D., Deming, D., Robinson, T. D., Tovar, G., Wolf, E. T., and Schwieterman, E.: Pale Orange Dots: The Impact of Organic Haze on the Habitability and detectability of earthlike exoplanets, Astrophys. J., 836, 19 pp., https://doi.org/10.3847/1538-4357/836/1/49, 2017.
Bach, L. T., Tamsitt, V., Gower, J., Hurd, C. L., Raven, J. A., and Boyd, P. W.: Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt, Nat. Commun., 12, 2556, https://doi.org/10.1038/s41467-021-22837-2, 2021.
Bacour, C., Bréon, F.-M., Gonzalez, L., Price, I., Muller, J.-P., and Straume, A. G.: Simulating multi-directional narrowband reflectance of the Earth's surface using ADAM (A surface reflectance database for ESA's Earth Observation Missions), Remote. Sens., 12, 1679, https://doi.org/10.3390/rs12101679, 2020.
Björn, L. O., Li, S., and Wang, Y.: Role of ultraviolet radiation in the origin of life, in: Photobiology: The Science of Light and Life, 3rd edn., edited by: Björn, L. O., Springer Science and Business Media New York, 415–420, ISBN 978-1-4939-1467-8 (Print), 978-1-4939-1468-5 (Online), https://doi.org/10.1007/978-1-4939-1468-5_27, 2015.
Blanco-Sacristán, J., Panigada, C., Tagliabue, G., Gentili, R., Colombo, R., Ladrón de Guevara, M., Maestre, F. T., and Rossini, M.: Spectral diversity successfully estimates the a-diversity of biocrust-forming lichens, Remote Sens., 11, 2942, https://doi.org/10.3390/rs11242942, 2019.
Blauw, A. N., Los, F. J., Huisman, J., and Peperzak, L.: Nuisance foam events and Phaeocystis globosa blooms in Dutch coastal waters analyzed with fuzzy logic, J. Marine Syst., 83, 115–126, https://doi.org/10.1016/j.jmarsys.2010.05.003, 2010.
Bottke, W. F. and Norman, M. D.: The late heavy bombardment, Annu. Rev. Earth Pl. Sc., 45, 619–47, https://doi.org/10.1146/annurev-earth-063016-020131, 2017.
Couradeau, E., Karaoz, U., Lim, H. C., Nunes da Rocha, U., Northen, T., Brodie, E., and Garcia-Pichel, F.: Bacteria increase arid-land soil surface temperature through the production of sunscreens, Nat. Commun., 7, 10373, https://doi.org/10.1038/ncomms10373, 2016.
Damer, B. and Deamer, D.: The hot spring hypothesis for an origin of life, Astrobiology, 20, 429–452, https://doi.org/10.1089/ast.2019.2045, 2020.
Delgado-Bonal, A.: Entropy of radiation: the unseen side of light, Sci. Rep., 7, 1642, https://doi.org/10.1038/s41598-017-01622-6, 2017.
Djokic, T., Van Kranendonk, M. J., Campbell, K. A., Walter, M. R., and Ward, C. R.: Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits, Nat. Commun., 8, 15263, https://doi.org/10.1038/ncomms15263, 2017.
Dodd, M. S., Papineau, D., Grenne, T., Slack, J. F., Rittner, M., Pirajno, F., O'Neil, J. L., and Crispin T. S.: Evidence for early life in Eart's okdest hydrothermal vent precipitates, Nature, 543, 60–64, https://doi.org/10.1038/nature21377, 2017.
Domagal-Goldman, S. D., Kasting, J. F., Johnston, D. T., and Farquhar, J.: Organic haze, glaciations and multiple sulfur isotopes in the Mid-Archean Era, Earth Planet. Sc. Lett., 269, 29–40, https://doi.org/10.1016/j.epsl.2008.01.040, 2008.
Hoashi, M., Bevacqua, D. C., Otake, T., Watanabe, Y., Hickman, A. H., Utsunomiya, S., and Ohmoto, H.: Primary haematite formation in an oxygenated sea 3.46 billion years ago, Nat. Geosci., 2, 301–306, https://doi.org/10.1038/ngeo465, 2009.
Homann, M.: Earliest life on Earth: Evidence from the Barberton Greenstone Belt, South Africa, Earth-Sci. Rev., 196, 102888, https://doi.org/10.1016/j.earscirev.2019.102888, 23 pp., 2019.
Izon, G., Zerkle, A. L., Zhelezinskaia, I., Farquhar, J., Newton, R. J., Poulton, S. W., Eigenbrode, J. L., and Claire, M. W.: Multiple oscillations in Neoarchaean atmospheric chemistry, Earth Planet. Sc. Lett., 431, 264–273, https://doi.org/10.1016/j.epsl.2015.09.018, 2015.
Jin, Z., Charlock, T. P., and Rutledge, K.: Analysis of broadband solar radiation and albedo over the ocean surface at COVE, J. Atmos. Ocean. Tech., 19, 1585–1601, 2002.
Jin, Z., Qiao, Y., Wang, Y., Fang, Y., and Yi, W.: A new parameterization of spectral and broadband ocean surface albedo, Opt. Express, 19, 26429–26442, https://doi.org/10.1364/OE.19.026429, 2011.
Kahru, M., Leppänen, J.-M., and Rud, O.: Cyanobacterial blooms cause heating of the sea surface, Mar. Ecol. Prog. Ser., 101, 1–7, https://doi.org/10.3354/meps101001, 1993.
Lepot, K.: Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon, Earth-Sci. Rev., 209, 103296, https://doi.org/10.1016/j.earscirev.2020.103296, 2020
Li, L., Solana, C., Canters, F., Chan, J. C.-W., and Kervyn, M.: Impact of environmental factors on the spectral characteristics of lava surfaces: Field spectrometry of basaltic lava flows on Tenerife, Canary Islands, Spain, Remote Sens., 7, 16986–17012, https://doi.org/10.3390/rs71215864, 2015.
Li, J., Li, T., Song, Q., and Ma, C.: Performance evaluation of Four ocean reflectance model, Remote Sens., 13, 2748, https://doi.org/10.3390/rs13142748, 2021.
Lutz, S., Anesio, A. M., Raiswell, R., Edwards, A., Newton, R. J., Gill, F., and Benning, L. G.: The biogeography of red snow microbiomes and their role in melting arctic glaciers, Nat. Commun., 7, 11968, https://doi.org/10.1038/ncomms11968, 2016.
Martin, W., Baross, J., Kelley, D., and Russell, M. J.: Hydrothermal vents and the origin of life, Nat. Rev. Microbiol., 6, 805–814, https://doi.org/10.1038/nrmicro1991, 2008.
Maruyama, S., Kurokawa, K., Ebisuzaki, T., Sawaki, Y., Suda, K., and Santosh, M.: Nine requirements for the origin of Earth's life: Not at the hydrothermal vent, but in a nuclear geyser system, Geosci. Front., 10, 1337–1357, https://doi.org/10.1016/j.gsf.2018.09.011, 2019.
Michaelian, K.: Thermodynamic dissipation theory for the origin of life, Earth Syst. Dynam., 2, 37–51, https://doi.org/10.5194/esd-2-37-2011, 2011.
Michaelian, K. and Simeonov, A.: Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum, Biogeosciences, 12, 4913–4937, https://doi.org/10.5194/bg-12-4913-2015, 2015.
Mojzsis, S. J., Arrhenius, G., McKeegan, K. D., Harrison, T. M., Nutman, A. P., and Friend, C. R. L.: Evidence for life on Earth before 3800 million years ago, Nature, 384, 55–59, https://doi.org/10.1038/384055a0, 1996.
Muñoz-Velasco, I., García-Ferris, C., Hernandez-Morales, R., Lazcano, A., Peret, J., and Becerra, A.: Methanogenesis on early stages of life: Ancient but not primordial, Origins Life Evol. B., 48, 407–420, https://doi.org/10.1007/s11084-018-9570-9, 2019.
Omran, A. and Pasek, M.: A constructive way to think about different hydrothermal environments for the origins of life, Life, 10, 36, https://doi.org/10.3390/life10040036, 2020.
Pavlov, A. A., Kasting, J. F., Eigenbrode, J. F., and Freeman, K. H.: Organic haze in Earth's early atmosphere: Source of low−13C Late Archean kerogens?, Geology, 29, 1003–1006, https://doi.org/10.1130/0091-7613(2001)029<1003:OHIESE>2.0.CO;2, 2001.
Qi, L. and Hu, C.: To what extent can Ulva and Sargassum be detected and separated in satellite imagery?, Harmful Algae, 103, 102001, https://doi.org/10.1016/j.hal.2021.102001, 2021.
Qi, L., Hu, C., Mikelsons, K., Wang, M., Lanced, V., Sun, S., Barnes, B. B., Zhao, J., and Van der Zandeg, D.: In search of floating algae and other organisms in global oceans and lakes, Remote Sens. Environ., 239, 111659, https://doi.org/10.1016/j.rse.2020.111659, 2020.
Rahlff, J., Stolle, C., Giebel, H.-A., Ili, N., Mustaffa, H., Wurl, O., and Herlemann, D. P. R.: Sea foams are ephemeral hotspots for distinctive bacterial communities contrasting sea-surface microlayer and underlying surface water, FEMS Microbiol. Ecol., 97, fiab035, https://doi.org/10.1093/femsec/fiab035, 2021.
Rutherford, W. A., Painter, T. H., Ferrenberg, S., Jayne Belnap, J., Okin, G. S., Flagg, C., and Reed, S. C.: Albedo feedbacks to future climate via climate change impacts on dryland biocrusts, Sci. Rep., 7, 44188, https://doi.org/10.1038/srep44188, 2017.
Teuling, A. J., Taylor, C. M., Meirink, J. F., Melsen, L. A., Miralles, D. G., van Heerwaarden, C. C., Vautard, R., Stegehuis, A. I., Nabuurs, G. J., and Vilà-Guerau de Arellano, J.: Observational evidence for cloud cover enhancement over western European forests, Nat. Commun., 8, 14065, https://doi.org/10.1038/ncomms14065, 2017.
Ueno, Y., Yamada, K., Yoshida, N., Maruyama, S., and Isozaki, Y.: Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era, Nature, 440, 516–519, https://doi.org/10.1038/nature04584, 2006.
Wu, W. and Liu, Y.: Radiation entropy flux and entropy production in the Earth system, Rev. Geophys., 48, RG2003, https://doi.org/10.1029/2008RG000275, 2010.
Xiao, B. and Bowker, M. A.: Moss-biocrusts strongly decrease soil surface albedo, altering land-surface energy balance in a dryland ecosystem, Sci. Total Environ., 741, 140425, https://doi.org/10.1016/j.scitotenv.2020.140425, 2020.
Yallop, M. L., Anesio, A. M., Perkins, R. G., Cook, J., Telling, J., Fagan, D., MacFarlane, J., Stibal, M., Barker, G., Bellas, C., Hodson, A., Tranter, M., Wadham, J., and Roberts, N. W: Photophysiology and albedo-changing potential of the ice algal community on the surface of the Greenland ice sheet, ISME J., 6, 2302–2313, https://doi.org/10.1038/ismej.2012.107, 2012.
Zhang, Y,-F., Wang, X.-P., Hu, R., Pan, Y.-X., and Zhang, H.: Variation of albedo to soil moisture for sand dunes and biological soil crusts in arid desert ecosystems, Environ. Earth. Sci., 71, 1281–1288, https://doi.org/10.1007/s12665-013-2532-7, 2013.
- Abstract
- Introduction: do living systems reduce the albedo of Earth?
- Ancient life
- Present vegetation compared to bare ground
- The temporal aspect
- The aquatic environment
- Ice and snow
- Conclusion
- Final comments
- Data availability
- Competing interests
- Disclaimer
- Acknowledgements
- Review statement
- References
thermodynamic imperative of dissipating the prevailing solar spectrum, as there are other ways for entropy increase in solar radiation. The biosphere may instead delay entropy production.
- Abstract
- Introduction: do living systems reduce the albedo of Earth?
- Ancient life
- Present vegetation compared to bare ground
- The temporal aspect
- The aquatic environment
- Ice and snow
- Conclusion
- Final comments
- Data availability
- Competing interests
- Disclaimer
- Acknowledgements
- Review statement
- References





