the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A saturating response of photosynthesis to an increasing leaf area index allows selective harvest of trees without affecting forest productivity
Ernst-Detlef Schulze
Konstantin Gregor
Issam Boukhris
Peter Högberg
Roland Irslinger
Phillip Papastefanou
Julia Pongratz
Anja Rammig
Riccardo Valentini
Christian Körner
Maintaining or increasing forest carbon sinks is considered essential for mitigating the rise in atmospheric CO2 concentrations. In contrast, harvesting trees is perceived as having negative consequences for both the standing biomass stocks and the carbon sink strength. However, the forest carbon sink needs to be examined from a forest stand canopy perspective, where assimilation predominantly occurs in temperate forests. Here we show that a threshold of leaf area exists beyond which additional leaves do not contribute to CO2 uptake. The associated biomass can be harvested without affecting the forest carbon uptake. Based on eddy covariance measurements, we show that CO2 uptake (gross primary production – GPP) and net ecosystem exchange (NEE) in temperate forests are of a similar magnitude in both unmanaged and sustainably managed forests, on the order of 1500–1600 for GPP and 542–483 for NEE. A threshold located between 3 and 4.5 m2 m−2 LAI (leaf area index) can be used for sustainable harvesting with regard to CO2 uptake. Simulations based on the LPJ-GUESS (Lund–Potsdam–Jena General Ecosystem Simulator) model reproduce the saturation of GPP and NEP and the convergence on the LAI threshold range. Accordingly, in temperate managed forests, trees can be harvested while maintaining a high tree biomass and carbon sink of the remaining stand. In this case, competition between neighboring trees in unmanaged forests is replaced by harvest management and provision of wood products. No difference in the LAI productivity response was observed between managed and unmanaged sites.
- Article
(1903 KB) - Full-text XML
-
Supplement
(987 KB) - BibTeX
- EndNote
At times of increasing global change and a demand for wood to replace fossil fuel products, it becomes of major importance to know the role of forest management and wood harvest in mitigating climate change. Following the EU definitions of storage and uptake, respectively (EE Agency, 2025), two major ways exist by which forests may contribute to efforts in climate mitigation: the storage of biomass at sites within the forest ecosystem and the storage of wood in products or their use for substitution of fossil fuel or carbon-intensive materials (Gregor et al., 2024). It is generally assumed that storage and C stocks can be sustained or increased only by increasing the area of forests or stopping wood procurement from forests (no management). However, halting management will probably have few long-term effects on the forest carbon sink and stocks at the landscape level, considering the environmental risks associated with climate change that strongly increase the chances of stand collapse (Roebroek et al., 2023). This is supported by Pretzsch et al. (2023), who observed that self-thinning losses could be equivalent to wood extraction by management. Luyssaert et al. (2011) also showed that management keeps forest stands close to but below self-thinning, albeit at different stand densities and volumes. Besides ensuring a sustained carbon sink, harvesting wood products can replace carbon-intensive materials, and the energy use of wood residues and end-of-life wood products can replace energy from fossil fuels (Cowie et al., 2021; Schulze et al., 2022). Thus, understanding the consequences of selective harvesting for the carbon balance and sink strength of forests is a key element in future projections of the role of forests in climate change mitigation.
Previous studies showed that forest productivity was not necessarily affected by selective harvesting (including various forms of thinning) across a large range of cutting intensities (Skovsgaard, 2009; Amiro et al., 2010; Peters et al., 2013; Bond-Lamberty et al., 2015; Noormets et al., 2015). Forestry studies such as Assmann (1970) likewise showed the fact that controlled thinnings have no long-term negative effects on productivity and could even increase it. The mechanisms involved in explaining the resilience of productivity to management are based on the enhanced productivity of the remaining trees. Reasons for this include, for example, improved light conditions, nutrient and water supply, and overall light use (Mund et al., 2010; Saunders et al., 2012; Sohn et al., 2016; del Campo et al., 2022). Compensatory contributions of subcanopy individuals can also be observed locally (Vesala et al., 2005). Several such factors and interaction pathways have been identified (e.g., Noormets et al., 2015, Fig. 1), but canopy density, as quantified by leaf area index (LAI, the cumulated area of leaves per ground square meter; m2 m−2), was not taken into consideration despite its key role in CO2 uptake.
Here, we introduce the link between photosynthesis and leaf area as a key element in this regulation at stand level. We hypothesize that LAI is not only the link between the atmosphere and the plant, but is also central to the response to management. LAI is indeed largely seen as a driver of both water and carbon fluxes (Reich, 2012; del Campo et al., 2022). Given its high nutrient demand, the production of leaves also affects the nutrient cycle (Ollinger et al., 2008) and is a potentially crucial driver of forests response to harvesting.
Harvesting inevitably results in a reduction of the amount of canopy leaves, best quantified by LAI. It can be assumed that a reduction of LAI would lead to a decrease in productivity. However, there are indications of a saturation of several canopy processes resulting in a nonlinear relation between leaf area index at stand level (Soimakallio et al., 2021) that make the response of productivity to disturbances complex (Glatthorn et al., 2017; Stuart-Haëntjens et al., 2015). Given the exponential light extinction with canopy depth, as described by Monsi and Saieki 1953 (see Hirose 2005), a rise in LAI must have diminishing returns in terms of light capture and CO2 assimilation. Concerning canopy conductance, Schulze et al., 1994 concluded to a saturation of around 3.5 m2 m−2. These elements suggest that productivity could also have a nonlinear response to reductions in LAI, and hence to management, while examined at stand level. Regardless of the mechanisms, however, the effects appear beyond a yet unknown level of biomass removal. A comparison across temperate forests beyond the site-level analyses is lacking.
The impact of harvest on the C cycle is clearly of major importance in the public discourse. It is thus necessary to determine the impact of harvesting on the fluxes of carbon in forests based on experimental data over a large gradient and to discuss the limits in the context of leaf area reduction. In particular, the interactions between management and LAI as well as their consequences for the carbon sink strength need to be determined in order to examine the consequences of wood harvesting for forest carbon sink strength. Here we intend to show that sustainable management replaces natural competition by regulating leaf area without affecting ecosystem fluxes in temperate forests. Based on observational data, literature, and modeling, we want to identify mechanistic reasons for this assumption and explore the possibilities of defining levels of sustainable partial cuttings from the perspective of carbon fluxes, which is key to designing forest management strategies able to maintain high biomass as well as forest C uptake over multiple cutting cycles. We use the LPJ-GUESS model to illustrate the diminishing returns of gross primary production (GPP) with increasing LAI in models as well.
2.1 Observational flux data based on eddy covariance measurements at the FLUXNET sites
In its 2015 release, FLUXNET represented 212 sites worldwide of eddy covariance. In order to measure the impact of management on the carbon fluxes, we have compiled flux data from the 29 FLUXNET sites (https://fluxnet.org/data/fluxnet2015-dataset/, last access: June 2025) that comprise 19 managed and 10 unmanaged sites (“unmanaged” is used in the sense of “intact” forests of Roebroek et al., 2023) with long-term measurements (i.e., >10 years, whenever possible) in temperate forests (Table S1 in the Supplement). Unfortunately, there is no site that covers unmanaged conifers. For each site we have compiled the forest type, stand type, and fluxes over their monitoring period. We completed these data with estimations of LAI during the period 2000–2020 and of the standing biomass.
Noticeably, selective harvesting took place at 11 of the managed sites during the period of flux monitoring, with several interventions being quite intensive (Table S3): for instance, 36 % LAI removal at the Fontainebleau site (FR) and 30 % LAI removal at the Bily Kriz site (CZ). Other managed sites have experienced interventions prior to the monitoring but not necessarily during the monitoring period, given the long periods of time separating interventions. Furthermore, during the period of flux monitoring, forests experienced repeated storm, drought, and heat events such as that of 2003, affecting ecosystem fluxes independent of management.
Further, we have compiled LAI estimations for the analyses for each of the FLUXNET sites. LAI measurements, however, are not standard across sites, and field measurements are not always available (fire sites had no field measurements). In this situation, remote-sensed estimations were used instead based on MCD15A3H version 6.1 MODIS data level 4 (see Table S1, with references for each estimation). Field measurements were based on hemispherical images with site-specific clumping factors (Gielen et al., 2018).
The eddy covariance method measures high-frequency atmospheric CO2, concentrations, and wind speed fluctuations which are then used to compute net ecosystem exchange (NEE). These measurements are then used to compute NEE with inherent uncertainties due to instrument limitations, atmospheric conditions, and data processing methods. Flux data were filtered based on USTAR threshold levels, following the method described by Pastorello et al. (2020), to exclude measurements taken under low-turbulence conditions. Errors were estimated 200 times using bootstrapping with different friction velocity values.
The fluxes of carbon exchanged between the forest ecosystem and the atmosphere are generally divided into components that are physiologically meaningful: the GPP corresponds to the photosynthesis of plants and the ecosystem respiration (Reco) releasing CO2. Reco consists of plant respiration (so-called autotrophic respiration) and respiration by heterotrophic organisms (so-called heterotrophic respiration). The NEE can be estimated by eddy covariance, and partitioning into the other elementary fluxes follows data-driven models (Valentini et al., 2000) and is expressed here following the biospheric convention (i.e., negative when CO2 leaves the atmosphere).
We compared the mean fluxes during the period of time available for managed and unmanaged sites. To test the significance of differences in NEE, we used the Wilcoxon rank test because the data were not distributed normally. GPP and Reco have a distribution that does not differ significantly from a normal distribution. The Mann–Whitney test was implemented to compare managed and unmanaged sites and works with unequal sample sizes. For GPP and Reco, their distributions are normal (Lasslop et al., 2008) but their variances are unequal, and the Welch t test was used instead. Subsequently, two-way analysis of variance for unbalanced designs was performed on the data to check whether the interaction between the management and the number of observations by the FLUXNET site has a significant effect on GPP, Reco, and NEE.
The relationship between GPP and LAI for the FLUXNET observational site was represented as a nonlinear asymptotic model. The fitting was based on the nonlinear fit function nls (“nls” standing for nonlinear least squares) in R. The pseudo-R2 value represents the proportion of variance that was explained by the model in lieu of R2, whose assumptions cannot be completely satisfied with nonlinear models (Schabenberger and Pierce, 2002). It was computed as pseudo-R, where var(yfit) is the variance of the predicted value (GPP here) and var(y) is the variance of the variable (GPP) within the dataset. All statistical analyses were performed in R version 4.3.2 (R Core Team, 2023).
2.2 Harvesting and carbon fluxes
Harvesting takes many forms in forest management and can have different intensities. It is defined in a general way as the removal of wood by tree cuttings of any kind, thus including tending, thinning (targeting either dominant or subdominant trees), and selective cuttings from either status. While short- and medium-term effects of selective harvesting are considered, this study will not cover the comparison of forest products with other bioenergy sources (product and energy substitution). In the following, clear-cutting, or final felling of a rotation, is treated separately from selective cuttings as it needs an assessment at the landscape or management unit scale. The measurement of carbon fluxes using the eddy covariance method is limited to the plot scale, with a footprint commonly of about 0.1 km2. Throughout this study, “harvesting” refers to practices of selective harvesting at low to moderate intensity as is common in temperate forests. For example, removal of harvest residuals is widely seen as negative because of the nutrient and soil carbon depletion it causes (Achat et al., 2015, Mayer et al., 2020).
2.3 Modeling analysis of the impact of an increasing LAI gradient on CO2 fluxes exchanged, using the process-based model
To investigate the impact of LAI on GPP, we used the LPJ-GUESS v4.1.1 dynamic global vegetation model (Smith et al., 2014; Nord et al., 2021) to simulate the main carbon fluxes (GPP, Reco, and NEP) at all of the eddy covariance sites used in the study. The ability of LPJ-GUESS to estimate LAI and GPP values worldwide has been proven in numerous studies (e.g., Vella et al., 2023; Ito et al., 2017; see also Fig. S2 in the Supplement). Therefore, the model is well suited for the analyses. LPJ-GUESS simulates a detailed vegetation structure (including cohorts of various ages) based on mechanistic modeling of ecosystem processes, including photosynthesis, establishment, growth, allocation, competition, water and nutrient limitation, and mortality of plant functional types (PFTs). The latter are represented by parameters defining plant characteristics, such as bioclimatic limits, growth form, or shade tolerance.
In the model, at the end of each year, cumulative net primary productivity is distributed among the leaf, root, sapwood, and heartwood compartments of a plant, based on allometric equations and allocation routines per year (Smith et al., 2014). The model belongs to the big-leaf family, representing the canopy as a single layer. This modeling is compatible with the space of the study. LAI is calculated as the product of the carbon mass of the leaves and the specific leaf area, the specific leaf area being a PFT parameter. LAI is computed proportionally to the phenology fraction of the PFTs, i.e., the fraction of potential leaf cover. The phenology of a PFT can be raingreen, summergreen, or evergreen. LAI is also influenced by the phenology: depending on the environmental conditions, the phenology fraction can depend on growing degree days and drought-stress-related model states. The amount of light taken up by the canopy, and thus contributing to carbon allocation, is governed by LAI, based on the Lambert–Beer law (Prentice et al., 1993) and assuming a site-specific surface leaf mass ratio not varying within the canopy. The model outputs stand-level LAI, taking into account the number of trees per area and the crown areas of the various cohorts. The photosynthesis model used in LPJ-GUESS is based on Collatz et al. (1991), which is a simplification of the Farquhar et al. (1980) model and the carbon allocation model based on Smith et al. (2001). Photosynthesis and respiration are calculated daily and accumulated towards the end of a year, allowing us to represent seasonal dynamics.
For the LAI analysis, we ran LPJ-GUESS until 2015 using daily climate data from the FLUXNET2015 sites, i.e., precipitation, temperature, and shortwave radiation. For each site, we prescribed the forest types as described in Table S2. We used 1000 years for the spinup period (to bring soil pools close to equilibrium) by detrending and recycling the first 10 years of each site's climate data. CO2 concentrations were taken from Büchner and Reyer (2022).
We used the default global parameterization of LPJ-GUESS with global PFTs, without any form of management.
Stochastic disturbance intervals were kept at default values, while fire was not simulated.
3.1 Saturated response of fluxes to LAI
Regular management actions were performed at most of the managed sites during the monitoring period with removals as high as 30 % of the stems for some sites during the monitoring period (Table S3). Managed sites are mostly age selection (forests stands composed of trees of similar ages, obtained from harvesting trees at a prescribed age), natural regeneration, and plantations. In the whole flux network, there is only one pair of managed and unmanaged sites, DE-Hai (Hainich, unmanaged) and DE-Lnf (Leinefelde, managed), representing Fagus sylvatica (L.) stands with similar stand densities or basal areas.
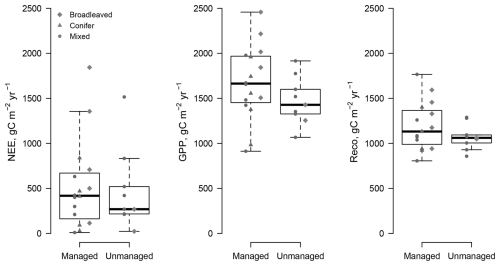
The data from the FLUXNET sites only show a response of GPP (the annual cumulated GPP) to LAI for LAI values less than (Fig. 1), but the GPP does not increase at a higher LAI. It is interesting to note that most managed forests operate above the range of saturating LAI with a mean of , despite harvesting. Likewise, the data show a saturation of GPP even at managed sites, with values reaching a plateau on the order of 1770 at LAI values as low as 2 m2 m−2. Based on the GPP–LAI regression, 95 % of the GPP (1680 ) is reached at a LAI of 2.7–4.0 m2 m−2, depending on the forest type. The exact location of the LAI saturation point can only be approximated given the uncertainty in both LAI and C flux data, which is larger in LAI than in fluxes (Fig. 1 and Table S1). The site at Parco Ticino (Italy) has been fertilized. It indicates the importance of nutrition in forest ecosystems as a GPP value above 1800 g C m2 yr−1 was reached at a low LAI (). However, even with fertilization, the fluxes and LAI values remain in the range of the other sites. Reco had a smaller overall variability than GPP () and showed no response to LAI. Likewise, there was no response to forest types. The net ecosystem exchange (the balance between photosynthesis and respiration; ) did not show any significant response to LAI, with values largely scattered around the mean ().
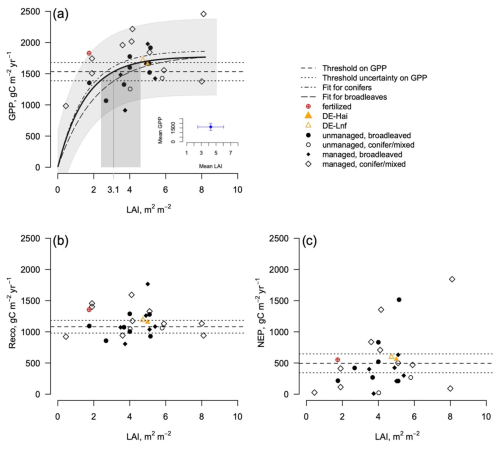
Figure 1Relationship between the GPP (a), the Reco (b), the NEP (, c), and the LAI at the eddy covariance sites (FLUXNET sites; see Tables S1 and S2) of both managed and unmanaged temperate forests per stand types. The dashed lines represent the mean and confidence interval of the GPP and NEP across all of the sites. The gray band represents the confidence interval of the regression at all of the sites and for all forest types. The fertilized site is identified (Parco Ticino), along with the DE-Hai (unmanaged) and DE-Lnf (managed) couple. The exponential models illustrate the tendencies (Table 1), and the ±10 % confidence intervals are displayed in gray.
The data represent a mixture of remotely sensed and field-based LAIs for the different forest types. Given the large variability among the sites, differences in fluxes for managed and unmanaged forests in Fig. 1 are not significant (Table 1).
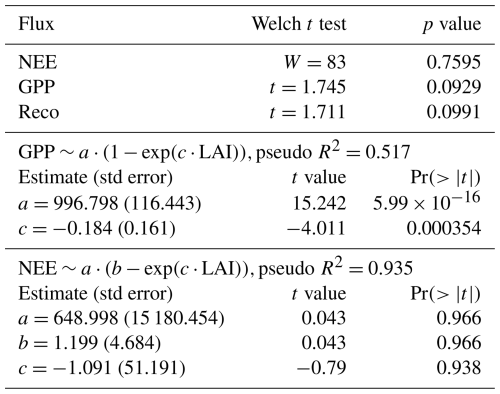
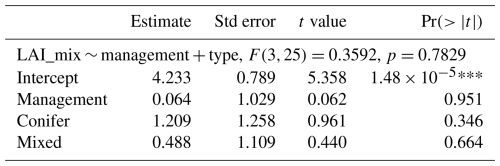
Table 1Effect of management type on the fluxes monitored at eddy covariance sites of the temperate Northern Hemisphere (N=29 FLUXNET sites, of which 18 are managed and 10 unmanaged, after the exclusion of the Parco Ticino site (IT) of fertilized Populus) and fit statistics of the nonlinear asymptotic models. Management is tested as a two-level fixed factor (managed or unmanaged) taken as a Wilcoxon rank test for NEE and a Welch t test for GPP, Reco, and LAI. Pseudo-R2 values were estimated from modeled and observed values (see the Methods section).
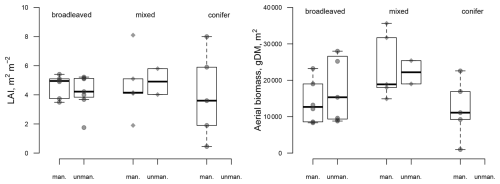
It is noteworthy that, under management, LAI was similar to that of unmanaged stands (4.74±1.33 for managed sites vs. for unmanaged sites, n.s. (not significant), despite the removal of parts of the canopy due to management in the past (Fig. 2). LAI was indeed strongly reduced during the monitoring period by thinnings ranging from 26 % to 36 % at four of the managed sites (Table S3). For instance, the low (3.6 m2 m−2) LAI value at site CS-BK1 (Picea abies L.) reflects the 26 % removal that occurred at the end of the monitoring period. The dynamic of LAI at the sites shows that the reduction in LAI by harvesting is limited to a few years following the harvesting (Fig. S1).
3.2 Responses of fluxes to sustainable harvesting: empirical evidence from eddy covariance
The FLUXNET-associated site data showed that past and current management has little influence on the aboveground biomass and LAI of the sites (Fig. 2). The highest biomass was reached at the old-growth Eucalyptus regnans (F. Muell.) site in Australia (Wallaby Creek site, with 36 106 g dry matter m−2). Unfortunately, there is no managed site of E. regnans for comparison. Otherwise, the range of values is very similar among the managed and unmanaged sites.
The comparison of the fluxes reveals that the net ecosystem exchange (the balance between photosynthesis and respiration) was not significantly different at managed and unmanaged sites ( for managed sites vs. and mean ± SD for unmanaged sites) over an observation period of more than a decade (Table 2). Management did not have a significant effect on GPP or NEP. As shown in Fig. 3, Reco and GPP tended to be higher at managed sites (Reco: at managed sites vs. 1079±98 at unmanaged sites; GPP: at managed sites vs. at unmanaged sites). The paired DE-Hai and DE-Lnf unmanaged sites had very similar values of both GPP (1709 at the managed site DE-Lnf vs. 1653 ) and NEP (1189 vs. 1155 ). We investigated whether the forest type had any influence on LAI or the fluxes, since conifers tend to have higher LAI values, with few exceptions. A linear model was fitted to the data and showed no significant influence of management or forest type (Table 2). Interactions between forest type and management were not significant either.
Table 2Estimation of the effect of management and forest type on LAI or the fluxes. Interactions (management × type) were tested and not found to be significant, and therefore they are not presented here.
3.3 Process-based model simulations: sensitivity to LAI
We applied the LPJ-GUESS process-based dynamic vegetation–terrestrial ecosystem model to further investigate the relationship between LAI, GPP, Reco, and NEP at each of the FLUXNET sites. According to the simulations, at a given site, GPP increased near linearly for LAI (), showing a clear inflection around this value (Fig. 4) but with some variability among the sites. The simulations illustrated the diminishing returns of a large LAI (LAI>4), whereby large cohorts with a high LAI contributed most to the total GPP due to the light extinction also represented in the model. Noticeably, the modeled LAI was always lower than the observed LAI, suggesting that the stands actually operate at LAI values in excess of the C-balance-optimal LAI. Reco followed a very similar pattern to GPP, albeit starting at higher values for very low LAI levels and having a smaller increase with LAI than GPP. GPP and Reco curves cross each other at different LAI values (between 1 and 3 m2 m−2) depending on the sites, at which point NEP becomes positive but shows a strong saturation afterwards, with no response at all to LAI. Thus, across all sites and regardless of the forest types, NEP becomes positive (forest acts as a sink) for LAI in excess of 3 m2 m−2, but, beyond 4.5 m2 m−2, increases in LAI do not result in increases in NEP.
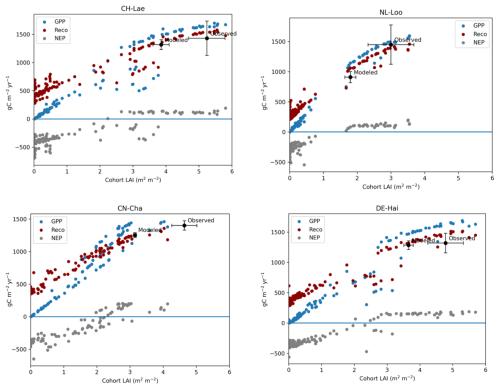
Figure 4Variations of GPP, NEP, and Reco along a gradient of LAI as modeled using LPJ-GUESS, shown for four sites with contrasted maximum LAI and forest types: CH-Lae for a mixed forest type with high LAI, NL-Loo for conifers with low LAI, broadleaf DE-Hai with high LAI, and broadleaf CN-Cha with low LAI. Each dot represents the fluxes of a particular tree cohort simulated at a given site. The model runs reveal that LAI in excess of 4 m2 m−2 does not promote GPP or NEP. NEP becomes positive (the forest acts as a sink) for LAI in excess of 3 m2 m−2, but, beyond 4 m2 m−2, increases in LAI do not result in increases in NEP.
With the introduction of the eddy covariance method, long time series of carbon fluxes became available over a variety of biomes, with most monitoring sites being under regular forest management (Franz et al., 2018). Based on these time series, our synthesis showed here that GPP and NEE remain largely unaffected by partial harvesting, as also reported by site-level analyses for several forest types and species (Granier et al., 2008; Launiainen et al., 2022; Lindroth et al., 2018; Pilegaard et al., 2011; Peichl et al., 2022; Vesala et al., 2005). These results are in agreement with the long-established empirical knowledge that stand productivity remains unaffected by thinnings when their intensity remains below a threshold (expressed in terms of stem density or basal area) (Assmann, 1970; Pretzsch and Schütze, 2009). Similarly, Vesala et al. (2005) observed no visible effects of thinnings on NEE despite the reduction in LAI from 8 to 6 m2 m−2 in a Scots pine (Pinus sylvestris L.) stand. Granier et al. (2008) reported for Fagus sylvatica (L.) stands no decrease in either NEE or GPP despite the thinning that decreased LAI from 7.4 to 4.8 m2 m−2. These results are in agreement with Herbst et al. (2015) and are confirmed by the global database of Luyssaert et al. (2007), which shows that managed forests globally achieved similar or even larger GPP than unmanaged forests.
The harvest effect on LAI appears to be short-term in temperate forests (del Campo et al., 2022), as also suggested by the available LAI time series of the sites studied here (Fig. S1). For instance, according to Granier et al. (2008), LAI in Fagus sylvatica stands was restored to its pre-thinning level within 2 years. Disturbances, particularly stand-replacing disturbances such as windthrow, fire, or clear-cuts, have a different dimension and need to be evaluated at the landscape scale. Our study deals with thinning operations where the main canopy is reduced but not removed, keeping LAI beyond or near its saturation threshold. This also justifies the choice of focusing on temperate forests where the lower species richness and age ranges may slow the recovery of carbon uptake after catastrophic events, in contrast to tropical forests (Brando et al., 2019). For boreal forests, the IBFRA report (Högberg et al., 2021) shows that biomass only increased significantly over the past decades in intensively managed landscapes but not in less intensively managed forest landscapes (i.e., landscapes with a high proportion of unmanaged forests). In the latter, large-scale disturbances such as wildfires caused losses of biomass and prevented a buildup of forest carbon stocks. In comparison, the biomass gain in unmanaged temperate forests is very small (Roebroek et al., 2023). Roerbroek et al. (2023) indeed suggest that betting on increasing the forests stocks is not only risky, given the increases in weather extremes, but also loses the societal benefit of wood products as well as the potential to store a portion of the C over the longer term.
We propose that most of the decoupling between selective harvesting and CO2 fluxes is mediated by the intrinsically nonlinear response of the dominant processes to LAI, with a saturation point reached at 3–4.5 m2 m−2 but with uncertainties around this value. The threshold itself may show some variability, e.g., related to plant functional types. The eddy covariance fluxes suggested a slightly higher relation between GPP and LAI for conifers than broadleaved (Fig. 1a). The model simulations likewise suggested varying levels of saturation depending on the sites. Further studies could help locate this threshold more precisely by increasing the number of observations and addressing the uncertainties, particularly those related to LAI estimates.
This nonlinear response, particularly the existence of a saturation point, is related to the existence of a fraction of the canopy leaf area which is not necessary for productivity but which also serves other functions, such as competition or redundancy in case of competition. In forest management, it is known that about one-third of the green-foliaged tree crown can be pruned to improve stem quality without affecting growth (Burschel and Huss, 2003). Diffuse light can penetrate deeper into the canopy and reach lower levels of leaves, but the gain in photosynthesis may not counterbalance the cost of producing and maintaining saturated canopies. The carbon balance of a living branch may be close to the light compensation point of photosynthesis and respiration (Schulze, 1970), with photosynthesis activity just at the level needed to keep a shaded branch alive. Similarly, in the simulations of the LPJ-GUESS model, small trees with a low LAI operate at a higher level of light extinction due to shadowing by bigger trees, which leads to very low GPP as no direct sunlight can reach any leaves (Fig. 4). Shadowing also leads to a reduction in Reco. However, a minimum maintenance respiration of the leaves is always needed to sustain functioning of the leaves.
While shade tolerance varies among species (Ameztegui et al., 2017), as reflected by different maximum LAI values (Valladares and Niinemets, 2008), the threshold for light compensation is probably very similar across forest types or species despite variations in the canopy structure. Accordingly, in our study, the species traits did not show significant correlations with either LAI or flux values. This suggests that increasing LAI beyond a demand-driven threshold has other functions, e.g., a competitive function with neighboring trees (Pretzsch and Schütze, 2009; Jucker et al., 2014), not only for light but also for nutrients (e.g., in a pre-emption strategy; Craine and Dybzinski, 2013), as a buffer against disturbance (e.g., herbivory) and a pool of nutrient reserves ready for rapid reallocation in case of sudden demand (Körner, 2009). Anten (2005) showed that canopy photosynthesis models predict LAI values greater than optimal values for photosynthesis and quote theoretical studies that conclude with a LAI always exceeding the physiologically optimal value for competitive purposes. Avoiding a neighbor increases the resources of water and nutrients for the dominant tree. This surplus fraction is temporarily diminished by selective harvesting, explaining the lack of a response of the main C fluxes at the canopy level across a wide range of LAIs. Accordingly, a moderate management can be seen as a substitution for self-thinning when forest stands are kept close to but below self-thinning density levels (Luyssaert et al., 2011).
These nonlinear relations of a variety of processes with LAI caused by a saturation of GPP and NEE at values around 3–4.5 m2 m−2 (see, e.g., Asner et al., 2003; Hirose, 2005) have long been known but were not previously related to the resilience to selective harvesting. This includes ecosystem respiration: according to Zhao et al. (2021), at high LAI, respiration – particularly heterotrophic respiration – increases more quickly than GPP, which results in a reduction in NPP for values larger than 5.6 m2 m−2. In our analysis, the model did not go so far as to project a negative impact of LAI on NEP, but the high cost of producing and maintaining leaves, and particularly shade leaves (Niinemets, 2010), largely suggests this. A similar result was obtained using the CASTANEA model, which reproduced the nonlinear responses of fluxes to LAI (Davi et al., 2006). In contrast, field measurements based on leaf collection, hemispherical photographs, or light transmission through plants frequently report values in excess of 5 m2 m−2 (e.g., Fig. 3) and even over 10 m2 m−2 in shade-tolerant species (Schulze et al., 1994; Asner et al., 2003; Law et al., 2001; Iio and Ito, 2014). Out of the 29 sites we studied here (Fig. 1), 16 display LAI values in excess of 4.5 m2 m−2. Issues related to the leaf clumping, requiring a specific correction factor as specified by the eddy site protocol (Gielen et al., 2018), add up to the already large uncertainties in the estimated LAI.
The lack of scaling between forest biomass and plant respiration (Piao et al., 2010) reflects the fact that the mass of live tissues – i.e., respiring tissues – is much smaller than that of the total biomass, basically scaling to the parenchyma fraction in sapwood volume and small branches only (Thurner et al., 2019). The disturbance-related increase in soil respiration, e.g., promoted by a short-term increase in root mortality (Raich and Nadelhoffer, 1989), could be comparable in magnitude to the reduction in plant respiration due to the amount of sapwood harvested and the reduced influx of fresh litter (Davidson et al., 2002) and explains the invariance of Reco. Surveying or modeling respiration has proven to be particularly difficult (Phillips et al., 2017; Ciais et al., 2021) and results in uncertainties, which also impact confidence in GPP estimates that could hide some effects. The lack of a response of Reco to LAI needs further investigation. Similarly, an in-depth analysis of the processes by which the C fluxes remain constant over a large range of LAI and the reason for the saturation based on the LPJ-GUESS model must still be conducted. Simulating management could help explain these behaviors. The LPJ-GUESS model may not be the best-suited one for such study though, because thinning induces many changes in the canopy structure and light conditions, which is difficult to represent in a big-leaf model. Its carbon allocation is not daily but seasonal, which could also be a limitation on fine-scale analyses. Despite these limitations, the model reproduced the saturation and confirmed that the stands generally function at LAI values exceeding this saturation point.
Unfortunately, the Hainich–Leinefelde Fagus sylvatica (L.) sites are the only paired managed and unmanaged sites within the flux network. The global eddy flux network was indeed strongly focused on climate as a main driver of fluxes rather than management. The management gradient represented by these sites is thus not complete. For instance, the intensity and types of management actions are not controlled. Although the unmanaged conifer sites are currently not monitored, the NEP values for unmanaged conifer stands reported in synthesis studies (Luyssaert et al., 2007) do not suggest that unmanaged conifer stands would behave differently and have a higher NEP than managed ones. We nevertheless highlight the potential of such paired studies and hope that research on management will be more integrated in the future in order to improve our understanding of its short-, medium-, and long-term impacts on the carbon balance of forests. This imbalance and low replication contributed to the difficulties in locating the saturation threshold. We therefore also underline the lack of common and frequent reporting on the aboveground biomass and annual LAI at the FLUXNET sites in harvested volumes whenever management interventions occur. Annual measurements of LAI and repeated study after disturbance should be considered. These critical data would strongly help measure the impact of management on the carbon cycle.
-
Based on observational and modeling evidence, it appears that LAI regularly exceeds the levels required to sustain carbon assimilation in naturally growing forest ecosystems.
-
Above its saturation value of 3–4.5 m2 m−2, additional increases in LAI are not linked to increased productivity but may contribute to other functions selected in evolution, such as competition with adjacent trees, resource storage, and buffering against herbivory.
-
We can explain the lack of impact of harvesting on the CO2 uptake by the existence of nonlinear processes that saturate around LAI values of 4.5 m2 m−2.
-
Selective harvesting does not reduce the forest carbon sink strength when LAI is maintained beyond its threshold.
-
This threshold can be used to define sustainable metrics for sustainable harvesting as those that do not impact the carbon sink strength of the forest stand.
-
Harmonized and periodic measurements of the forest carbon stock and LAI, together with the harvesting impacts on these, should be promoted at flux sites.
The data presented and analyzed in this study are available directly from the Supplement in Tables S1–S3. These tables also contain references to data sources. The figures were created with R version 4.3.2 (https://www.R-project.org/, R Core Team, 2023) .
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-4729-2025-supplement.
Conceptualization: OB, EDS, and CK. Methodology: OB and EDS. Writing – original draft preparation: OB and EDS. All of the authors contributed to the writing and reviewed the manuscript.
At least one of the (co-)authors is a member of the editorial board of Biogeosciences. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This work was supported by a grant from the Ministry of Research, Innovation and Digitization (CNCS-UEFISCDI, project no. PN-III-P4-PCE-2021-1677) within PNCDI III. KG acknowledges funding by the Bavarian State Ministry of Science and the Arts in the framework of the Bavarian Climate Research Network (bayklif) through its BLIZ project (grant no. 7831-26625-2017, http://www.bayklif-bliz.de, last access: June 2025). Riccardo Valentini and Issam Boukhris are supported by AGRITECH – PNRR (Italian National Plan of Recovery and Resilience), code no. CN00000022 WP 4.3.3. The authors are very grateful to Susan Trumbore, Jean-Daniel Bontemps, and two anonymous reviewers for their comments and suggestions on the manuscript.
This research has been supported by the Unitatea Executiva pentru Finantarea Invatamantului Superior, a Cercetarii, Dezvoltarii si Inovarii (grant-no. PN-III-P4-PCE-2021-1677), the Bavarian State Ministry of Science and the Arts in the context of the Bavarian Climate Research Network (bayklif) through its BLIZ project (grant no. 7831-26625- 2017, https://www.bayklif-bliz.de (last access: 3 August 2025), and AGRITECH – PNRR (Italian National Plan of Recovery and Resilience), identification code CN00000022 WP 4.3.3.
This paper was edited by Paul Stoy and reviewed by two anonymous referees.
Achat, D. L., Deleuze, C., Landmann, G., Pousse, N., Ranger, J., and Augusto, L.: Quantifying consequences of removing harvesting residues on forest soils and tree growth – a meta-analysis, Forest Ecol. Manag., 348, 124–141, 2015.
Ameztegui, A., Paquette, A., Shipley, B., Heym, M., Messier, C., and Gravel, D.: Shade tolerance and the functional trait: Demography relationship in temperate and boreal forests, Funct. Ecol., 31, 821–830, 2017.
Amiro, B. D., Barr, A. G., Barr, J. G., Black, T. A., Bracho, R., Brown, M., Chen, J., Clark, K. L., Davis, K. J., Desai, A. R., Dore, S., Engel, V., Fuentes, J. D., Goldstein, A. H., Goulden, M. L., Kolb, T. E., Lavigne, M. B., Law, B. E., Margolis, H. A., Martin, T., McCaughey, J. H., Misson, L., Montes-Helu, M., Noormets, A., Randerson, J. T., Starr, G., and Xiao, J.: Ecosystem carbon dioxide fluxes after disturbance in forests of North America, J. Geophys. Res.-Biogeo., 115, G00K02, https://doi.org/10.1029/2010JG001390, 2010.
Anten, N. P.: Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence, Ann. Bot., 95, 495–506, 2005.
Asner, G. P., Scurlock, J. M., and A. Hicke, J.: Global synthesis of leaf area index observations: implications for ecological and remote sensing studies, Global Ecol. Biogeogr., 12, 191–205, 2003.
Assmann, E.: The principles of forest yield study: studies in the organic production, structure, increment and yield of forest stands, Pergamon Press, Oxford, 506, ISBN 139780080066585, 1970.
Bond-Lamberty, B., Fisk, J. P., Holm, J. A., Bailey, V., Bohrer, G., and Gough, C. M.: Moderate forest disturbance as a stringent test for gap and big-leaf models, Biogeosciences, 12, 513–526, https://doi.org/10.5194/bg-12-513-2015, 2015.
Brando, P. M., Silvério, D., Maracahipes-Santos, L., Oliveira-Santos, C., Levick, S. R., Coe, M. T., Migliavacca, M., Balch, J. K., Macedo, M. N., Nepstad, D. C., and Maracahipes, L.: Prolonged tropical forest degradation due to compounding disturbances: Implications for CO2 and H2O fluxes, Glob. Change Biol., 25, 2855–2868, 2019.
Burschel, P. and Huss, J.: Grundriss des Waldbaus ein Leitfaden für Stadium und Praxis, 3rd unchanged edn., Eugen Ulmer Verlag, Stuttgart (Hohenheim), ISBN 9783800145706, 2003.
Büchner, M. and Reyer, P.: ISIMIP3b atmospheric composition input data (v1.1), ISIMIP Repository [data set], https://doi.org/10.48364/ISIMIP.482153.1, 2022.
Ciais, P., Yao, Y., Gasser, T., Baccini, A., Wang, Y., Lauerwald, R., Peng, S., Bastos, A., Li, W., Raymond, P. A., and Canadell, J. G.: Empirical estimates of regional carbon budgets imply reduced global soil heterotrophic respiration, Nat. Sci. Rev., 8, nwaa145, https://doi.org/10.1093/nsr/nwaa145, 2021.
Collatz, G. J., Ball, J. T., Grivet, C., and Berry, J. A.: Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary layer, Agr. For. Met., 54, 107–136, 1991.
Cowie, A. L., Berndes, G., Bentsen, N. S., Brandao, M., Cherubini, F., Egnell, G., Brendan, G., Guvstavsson, L., Hanwinkel, M., Harris, Z., Johnsson, F., Junginger, M., Kline, K., Koponen, K., Koppejan, J., Kraxner, F., Lamers, P., Majer, S., Marland, E., Nabuurs, G.-J., Pelkmans, L. Sathre, R., Schaub, M., Tattersal Smith, C., Soimakallio, S., Van der Hilst, F., Woods, J., and Ximenes, F. A.: Applying a science-based systems perspective to dispel misconceptions about climate effects of forest bioenergy, GCB Bioenergy, 13, 1210–1231, 2021.
Craine, J. M. and Dybzinski, R.: Mechanisms of plant competition for nutrients, water and light, Funct. Ecol., 27, 833–840, 2013.
Davi, H., Bouriaud, O., Dufrêne, E., Soudani, K., Pontailler, J. Y., Le Maire, G., François, C., Bréda, N., Granier, A., and Le Dantec, V.: Effect of aggregating spatial parameters on modelling forest carbon and water fluxes, Agr. Forest Meteorol., 139, 269–287, 2006.
Davidson, E. A., Savage, K., Bolstad, P., Clark, D. A., Curtis, P. S., Ellsworth, D. S., Hanson, P. J., Law, B. E., Luo, Y., Pregitzer, K. S., and Randolph, J. C.: Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements, Agr. Forest Meteorol., 113, 39–51, 2002.
del Campo, A. D., Otsuki, K., Serengil, Y., Blanco, J. A., Yousefpour, R., and Wei, X.: A global synthesis on the effects of thinning on hydrological processes: Implications for forest management, Forest Ecol. Manag., 519, 120324, https://doi.org/10.1016/j.foreco.2022.120324, 2022.
EE Agency: Carbon sink, https://www.eea.europa.eu/help/glossary/eea-glossary/carbon-sink#:~:text=Forests%20and%20other%20ecosystems%20that,atmosphere%20and%20offsetting%20CO2%20emissions (last access: 21 August 2023), 2025.
Farquhar, G. D., von Caemmerer, S. V., and Berry, J. A.: A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90, 1980.
Franz, D., Acosta, M., Altimir, N., Arriga, N., Arrouays, D., Aubinet, M., Aurela, M., Ayres, E., López-Ballesteros, A., Barbaste, M., and Berveiller, D.: Towards long-term standardised carbon and greenhouse gas observations for monitoring Europe's terrestrial ecosystems: a review, Int. Agrophys., 32, 439–455, 2018.
Gielen, B., Acosta, M., Altimir, N., Buchmann, N., Cescatti, A., Ceschia, E., Fleck, S., Hortnagal, L., Klumpp, K., Kolari, P., and Lohile, A.: Ancillary vegetation measurements at ICOS ecosystem stations, Int. Agrophys., 32, 645–664, 2018.
Glatthorn, J., Pichler, V., Hauck, M., and Leuschner, C.: Effects of forest management on stand leaf area: Comparing beech production and primeval forests in Slovakia, Forest Ecol. Manag., 389, 76–85, 2017.
Granier, A., Bréda, N., Longdoz, B., Gross, P., and Ngao, J.: Ten years of fluxes and stand growth in a young beech forest at Hesse, North-Eastern France, Ann. For. Sci., 65, 704, https://doi.org/10.1051/forest:2008052, 2008.
Gregor, K., Krause, A., Reyer, C. P., Knoke, T., Meyer, B. F., Suvanto, S., and Rammig, A.: Quantifying the impact of key factors on the carbon mitigation potential of managed temperate forests, Carbon Balance Manage., 19, 10, https://doi.org/10.1186/s13021-023-00247-9, 2024.
Herbst, M., Mund, M., Tamrakar, R., and Knohl, A.: Differences in carbon uptake and water use between a managed and an unmanaged beech forest in central Germany, Forest Ecol. Manag., 355, 101–108, 2015.
Hirose, T.: Development of the Monsi–Saeki theory on canopy structure and function, Ann. Bot., 95, 483–494, 2005.
Högberg, P., Ceder, L. A., Astrup, R., Binkley, D., Dalsgaard, L., Egnell, G., Filipchuk, A., Genet, H., Ilintsev, A., Kurz, W. A., and Laganière, J.: Sustainable boreal forest management challenges and opportunities for climate change mitigation, Swedish Forest Agency Report No. 11, ISBN 978-91-986297-3-6, 2021.
Iio, A. and Ito, A.: A global database of field-observed leaf area index in woody plant species, Oak Ridge National Laboratory Distributed Active Archive Center (ORNL DAAC), NASA Earth Science Data and Information System (EOSDIS) [data set], 1932–2011, https://doi.org/10.3334/ORNLDAAC/1231, 2014.
Ito, A., Nishina, K., Reyer, C. P., François, L., Henrot, A. J., Munhoven, G., Jacquemin, I., Tian, H., Yang, J., Pan, S., and Morfopoulos, C.: Photosynthetic productivity and its efficiencies in ISIMIP2a biome models: benchmarking for impact assessment studies, Environ. Res. Lett., 12, 085001, https://doi.org/10.1088/1748-9326/aa7a19, 2017.
Jucker, T., Bouriaud, O., Avacaritei, D., Danila, I., Duduman, G., Valladares, F., and Coomes, D. A.: Competition for light and water play contrasting roles in driving diversity–productivity relationships in Iberian forests, J. Ecol., 102, 1202–1213, 2014.
Körner, C.: Responses of humid tropical trees to rising CO2, Annu. Rev. Ecol. Syst., 40, 61–79, 2009.
Launiainen, S., Katul, G. G., Leppä, K., Kolari, P., Aslan, T., Grönholm, T., Korhonen, L., Mammarella, I., and Vesala, T.: Does growing atmospheric CO2 explain increasing carbon sink in a boreal coniferous forest?, Glob. Change Biol., 28, 2910–2929, 2022.
Lasslop, G., Reichstein, M., Kattge, J., and Papale, D.: Influences of observation errors in eddy flux data on inverse model parameter estimation, Biogeosciences, 5, 1311–1324, https://doi.org/10.5194/bg-5-1311-2008, 2008.
Law, B. E., Cescatti, A., and Baldocchi, D. D.: Leaf area distribution and radiative transfer in open-canopy forests: implications for mass and energy exchange, Tree Physiol., 21, 777–787, 2001.
Lindroth, A., Holst, J., Heliasz, M., Vestin, P., Lagergren, F., Biermann, T., Cai, Z., and Mölder, M.: Effects of low thinning on carbon dioxide fluxes in a mixed hemiboreal forest, Agr. Forest Meteorol., 262, 59–70, 2018.
Luyssaert, S., Inglima, I., Jung, M., Richardson, A. D., Reichstein, M., Papale, D., Piao, S. L., Schulze, E. D., Wingate, L., Matteucci, G., and Aragao, L. E.: CO2 balance of boreal, temperate, and tropical forests derived from a global database, Glob. Change Biol., 13, 2509–2537, 2007.
Luyssaert, S., Hessenmöller, D., von Lüpke, N., Kaiser, S., and Schulze, E. D.: Quantifying land use and disturbance intensity in forestry, based on the self‐thinning relationship, Ecol. Appl., 21, 3272–3284, https://doi.org/10.1890/10-2395.1, 2011.
Mayer, M., Prescott, C., Abaker, W., Augusto, L., Cécillon, L., Ferreira, G., and Vesterdal, L.: Influence of forest management activities on soil organic carbon stocks: a knowledge synthesis, Forest Ecol. Manag., 466, 118127, https://doi.org/10.1016/j.foreco.2020.118127, 2020.
Mund, M., Kutsch, W. L., Wirth, C., Kahl, T., Knohl, A., Skomarkova, M. V., and Schulze, E.-D.: The influence of climate and fructification on the inter-annual variability of stem growth and net primary productivity in an old-growth, mixed beech forest, Tree Physiol., 30, 689–704, 2010.
Niinemets, Ü.: A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance, Ecol. Res., 25, 693–714, 2010.
Noormets, A., Epron, D., Domec, J.-C., McNulty, S., Fox, T., Sun, G., and King, J.: Effects of forest management on productivity and carbon sequestration: A review and hypothesis, Forest Ecol. Manag., 355, 124–140, 2015.
Nord, J., Anthoni, P., Gregor, K., Gustafson, A., Hantson, S., Lindeskog, M., Meyer, B., Miller, P., Nieradzik, L., Olin, S., Papastefanou, P., Smith, B., Tang, J., and Wårlind, D.: LPJ-GUESS Release v4, 1.1 model code, Zenodo [code], https://doi.org/10.5281/zenodo.8065737, 2021.
Ollinger, S. V., Richardson, A. D., Martin, M. E., Hollinger, D. Y., Frolking, S. E., Reich, P. B., Plourde, L. C., Katul, G. G., Munger, J. W., Oren, R., and Smith, M. L.: Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks, P. Natl. Acad. Sci. USA, 105, 19336–19341, 2008.
Pastorello, G., Trotta, C., Canfora, E., Chu, H., Christianson, D., Cheah, Y. W., Poindexter, C., Chen, J., Elbashandy, A., Humphrey, M., and Isaac, P.: The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data, Sci. Data, 7, 225, https://doi.org/10.1038/s41597-020-0534-3, 2020.
Peichl, M., Martínez-García, E., Fransson, J. E., Wallerman, J., Laudon, H., Lundmark, T., and Nilsson, M. B.: Landscape-variability of the carbon balance across managed boreal forests, Glob. Change Biol., 1119–1132, https://doi.org/10.1111/gcb.16534, 2022.
Peters, E. B., Wythers, K. R., Bradford, J. B., and Reich, P. B.: Influence of disturbance on temperate forest productivity, Ecosystems, 16, 95–110, 2013.
Phillips, C. L., Bond-Lamberty, B., Desai, A. R., Lavoie, M., Risk, D., Tang, J., Todd-Brown, K., and Vargas, R.: The value of soil respiration measurements for interpreting and modeling terrestrial carbon cycling, Plant Soil, 413, 1–25, 2017.
Piao, S., Luyssaert, S., Ciais, P., Janssens, I. A., Chen, A., Cao, C., Fang, J., Friedlingstein, P., Luo, Y., and Wang, S.: Forest annual carbon cost: A global-scale analysis of autotrophic respiration, Ecology, 91, 652–661, 2010.
Pilegaard, K., Ibrom, A., Courtney, M. S., Hummelshøj, P., and Jensen, N. O.: Increasing net CO2 uptake by a Danish beech forest during the period from 1996 to 2009, Agr. Forest Meteorol., 151, 934–946, 2011.
Prentice, I. C., Sykes, M. T., and Cramer, W.: A simulation model for the transient effects of climate change on forest landscapes, Ecol. Model., 65, 51–70, 1993.
Pretzsch, H., del Río, M., Arcangeli, C., Bielak, K., Dudzinska, M., Forrester, D. I., Ledermann, T., Matthews, R., Nagel, R., Ningre, F.: Competition-based mortality and tree losses. An essential component of net primary productivity, Forest Ecol. Manag., 544, 121204, https://doi.org/10.1016/j.foreco.2023.121204, 2023.
Pretzsch, H. and Schütze, G.: Transgressive overyielding in mixed compared with pure stands of Norway spruce and European beech in Central Europe: evidence on stand level and explanation on individual tree level, Eur. J. For. Res., 128, 183–204, 2009.
R Core Team: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 21 August 2023), 2023.
Raich, J. W. and Nadelhoffer, K. J.: Belowground carbon allocation in forest ecosystems: global trends, Ecology, 70, 1346–1354, 1989.
Reich, P. B.: Key canopy traits drive forest productivity, P. R. Soc. B, 279, 2128–2134, 2012.
Roebroek, C. T., Duveiller, G., Seneviratne, S. I., Davin, E. L., and Cescatti, A.: Releasing global forests from human management: How much more carbon could be stored?, Science, 380, 749–753, 2023.
Saunders, M., Tobin, B., Black, K., Gioria, M., Nieuwenhuis, M., and Osborne, B.: Thinning effects on the net ecosystem carbon exchange of a Sitka spruce forest are temperature-dependent, Agr. Forest Meteorol., 157, 1–10, 2012.
Schabenberger, O., Pierce, F. J.: Contemporary statistical models for the plant and soil sciences, Taylor and Francis, CRC Press, Books, https://doi.org/10.1201/9781420040197, 2002.
Schulze, E.-D.: Der CO2-gaswechsel der Buche (Fagus silvatica L.) in Abhängigkeit von den Klimafaktoren im Freiland, Flora, 159, 177–232, 1970.
Schulze, E. D., Bouriaud, O., Irslinger, R., and Valentini, R.: The role of wood harvest from sustainably managed forests in the carbon cycle, Ann. For. Sci., 79, 1–13, 2022.
Schulze, E.-D., Kelliher, F. M., Körner, C., Lloyd, J., and Leuning, R.: Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: a global ecology scaling exercise, Annu. Rev. Ecol. Syst., 25, 629–662, 1994.
Skovsgaard, J. P.: Analysing effects of thinning on stand volume growth in relation to site conditions: a case study for even-aged sitka spruce (Picea sitchensis (bong.) carr.), Forestry, 82, 87–104, 2009.
Smith, B., Prentice, I. C., and Sykes, M. T.: Representation of vegetation dynamics in the modelling of terrestrial ecosystems: comparing two contrasting approaches within European climate space, Global Ecol. Biogeogr., 10, 621–637, https://doi.org/10.1046/j.1466-822X.2001.t01-1-00256.x, 2001.
Smith, B., Wårlind, D., Arneth, A., Hickler, T., Leadley, P., Siltberg, J., and Zaehle, S.: Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model, Biogeosciences, 11, 2027–2054, https://doi.org/10.5194/bg-11-2027-2014, 2014.
Sohn, J. A., Saha, S., and Bauhus, J.: Potential of forest thinning to mitigate drought stress: A meta-analysis, Forest Ecol. Manag., 380, 261–273, 2016.
Soimakallio, S., Kalliokoski, T., Lehtonen, A., and Salminen, O.: On the trade-offs and synergies between forest carbon sequestration and substitution, Mitig. Adapt. Strat. Gl., 26, 1–17, 2021.
Stuart-Haëntjens, E. J., Curtis, P. S., Fahey, R. T., Vogel, C. S., and Gough, C. M.: Net primary production of a temperate deciduous forest exhibits a threshold response to increasing disturbance severity, Ecology, 96, 2478–2487, 2015.
Thurner, M., Beer, C., Crowther, T., Falster, D., Manzoni, S., Prokushkin, A., and Schulze, E.-D.: Sapwood biomass carbon in northern boreal and temperate forests, Global Ecol. Biogeogr., 28, 640–660, 2019.
Valentini, R., Matteucchi, G., Dolman, H., Schulze, E.-D., Reb- mann, C., Moors, E. J., Granier, A., Gross, P., Jensen, N. O., Pilgaard, K., Lindroth, A., Grelle, A., Bernhofer, C., Grünwald, T., Aubinet, M., Ceulemans, R., Kowalski, A. S., Vesala, T., Rannik, Ü., Berbigier, P., Lousteau, D., Gudmundsson, J., Thorgairsson, H., Ibrom, A., Morgenstern, K., Clement, R., Moncrieff, J., Montagnani, L., Minerbi, S., and Jarvis, P. G.: Respiration as the main determinant of carbon balance in European forests, Nature, 404, 861–865, 2000.
Valladares, F. and Niinemets, Ü.: Shade tolerance, a key plant feature of complex nature and consequences, Annu. Rev. Ecol. Syst., 39, 237–257, 2008.
Vella, R., Forrest, M., Lelieveld, J., and Tost, H.: Isoprene and monoterpene simulations using the chemistry–climate model EMAC (v2.55) with interactive vegetation from LPJ-GUESS (v4.0), Geosci. Model Dev., 16, 885–906, https://doi.org/10.5194/gmd-16-885-2023, 2023.
Vesala, T., Suni, T., Rannik, U., Keronen, P., Markkanen, T., Se- vanto, S., Gronholm, T., Smolander, S., Kulmala, M., Ilves- niemi, H., Ojansuu, R., Uotila, A., Levula, J., Makela, A., Pumpanen, J., Kolari, P., Kulmala, L., Altimir, N., Berninger, F., Nikinmaa, E., and Hari, P.: Effect of thinning on surface fluxes in a boreal forest, Global Biogeochem. Cy., 19, GB2001, https://doi.org/10.1029/2004gb002316, 2005.
Zhao, W., Tan, W., and Li, S.: High leaf area index inhibits net primary production in global temperate forest ecosystems, Environ. Sci. Pollut. Res. Int., 28, 22602–22611, 2021.