the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Nitrogen dynamics and nitrate stable isotopes indicate nitrogen loss in the Bay of Bengal
Kirstin Dähnke
Tina Sanders
Jan Penopp
Hermann W. Bange
Rena Czeschel
Birgit Gaye
Oxygen-minimum zones (OMZs) play an important role in the global oceanic nitrogen cycle because they account for 20 % to 40 % of the global loss of bioavailable nitrogen despite covering only about 1 % of the global ocean volume. The intermediate waters of the Bay of Bengal (BoB) host one of the most pronounced OMZs with near-anoxic conditions. However, it has not yet been recognized as a site with significant nitrate reduction. In this study, we examined the nitrogen-cycling processes in the East Equatorial Indian Ocean (EEIO) and the BoB by measuring water column properties, including temperature, salinity, oxygen, and nutrient concentrations, as well as nitrate isotope signatures, collected during the SO305 BIOCAT-IIOE2 cruise in April and May 2024. Potential temperature and salinity profiles showed distinct water masses and limited mixing between the BoB and the EEIO at 5° N.
Nitrate stable isotope depth profiles varied significantly, driven by water mass distribution below 300 m and in situ fractionation above 300 m. Phytoplankton uptake acts as a nitrate sink in the surface waters, showing a significant isotopic enrichment and nitrogen deficit. In subsurface waters, nitrification was observed, primarily through regenerative production using previously assimilated biomass rather than newly fixed nitrogen from N2 fixation. Within the OMZ of the BoB, we identified a persistent nitrogen deficit and slightly enriched nitrate isotopes between 100 and 300 m, indicating nitrogen loss, which we attributed to anammox as the dominant nitrogen loss pathway in the BoB.
- Article
(7237 KB) - Full-text XML
-
Supplement
(368 KB) - BibTeX
- EndNote
Nitrogen is an essential nutrient for all organisms. However, in its most abundant form, unreactive dinitrogen gas (N2), it is unavailable for most organisms. Through N2 fixation, N2 is converted into organic nitrogen, making it bioavailable (Gruber, 2008). This bioavailable nitrogen is removed via denitrification and anammox (Fig. 1), creating N2. The balance between N2 fixation and nitrogen loss controls the availability of bioavailable nitrogen in the ocean and, consequently, marine productivity (Gruber, 2008).
Denitrification is a stepwise dissimilatory process, which can either be heterotrophic or autotrophic, that reduces nitrate () to N2 via nitrite (), nitric oxide (NO), and nitrous oxide (N2O) (Knowles, 1982; Tiedje, 1988). During anammox (anaerobic ammonium oxidation), ammonium and nitrite are combined to produce N2 (Strous et al., 1999; Trimmer et al., 2003). In addition to these loss processes, bioavailable nitrogen can be recycled. During assimilation, phytoplankton uses either nitrate or ammonium () to build biomass. This biomass is transformed back into ammonium through remineralization, which can subsequently be further oxidized into nitrate via nitrification, with nitrite as an intermediate product (Fig. 1; Gruber, 2008).
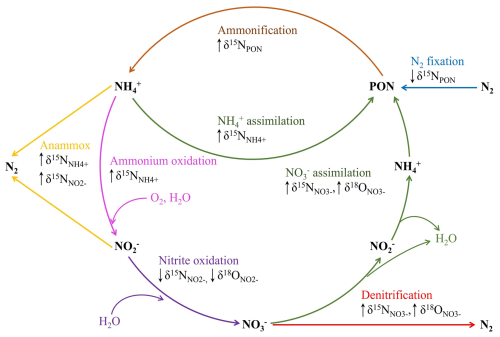
Figure 1Schematic overview of the nitrogen cycle with isotopic changes caused by N and O fractionation, adapted from Casciotti (2016). Abbreviations: particulate organic nitrogen – PON, ammonium – , nitrite – , nitrate – , dinitrogen – N2, water – H2O, and oxygen – O2.
Nitrogen loss processes are inhibited by the presence of oxygen, which is why oxygen-minimum zones (OMZs, with oxygen concentrations <20 µM) account for about 30 % of fixed nitrogen loss despite only covering 1 % of the global ocean (DeVries et al., 2012). Anammox has, in some cases, been observed to tolerate oxygen concentrations up to 20 µM (Kalvelage et al., 2011) and denitrification up to oxygen concentrations of 6 µM (Bristow et al., 2016). However, in OMZ waters, the rates of both processes increase only at oxygen concentrations <1 µM. However, the oxygen thresholds of denitrification and anammox are still associated with a high degree of uncertainty (Bristow et al., 2016; Dalsgaard et al., 2014; Kalvelage et al., 2011; Rixen et al., 2020). This also applies to nitrification, which usually occurs under oxic conditions but can also occur in niches with extremely low oxygen concentrations (Sun et al., 2021, 2023). A profound knowledge of the nitrogen turnover processes in OMZs and their regulation by ambient dissolved oxygen concentrations is essential to understand the global oceanic nitrogen cycle.
The northern East Indian Ocean, with its marginal sea, the Bay of Bengal (BoB), hosts one of the most pronounced OMZs in intermediate waters worldwide, with oxygen concentrations close to anoxic conditions (Rixen et al., 2020; Sridevi and Sarma, 2020). Following its discovery (Wyrtki, 1971), the BoB's OMZ has been well described in the literature (Deuser, 1975; Naqvi et al., 1978; Sen Gupta et al., 1977). To date, no study has measured signs of significant nitrogen loss in this area (Howell et al., 1997; Rao et al., 1994; Sardessai et al., 2007; Sarma et al., 2013), although, in 2017, Bristow et al. (2017) estimated a small nitrogen loss of ∼1.7 Tg yr−1. They hypothesized that more intense nitrogen loss was likely to be inhibited by variable oxygen concentrations within the BoB (Bristow et al., 2017; Johnson et al., 2019). Currently, it is being discussed whether the BoB is on the verge of becoming functionally anoxic and may thus evolve towards a significant oceanic nitrogen sink in the future (Bristow et al., 2017; Rixen et al., 2020; Sarma and Udaya Bhaskar, 2018; Sridevi and Sarma, 2020).
Natural variations in nitrate dual isotopes (nitrogen and oxygen) are a commonly used tool to study the marine nitrogen cycle. Nitrogen turnover processes usually preferably use lighter isotopes, leading to an enrichment of the remaining substrate pool (Fig. 1). The magnitude of the enrichment is called the isotope effect and is process specific. For the isotope effect, we follow the updated notation of Casciotti et al. (2003). The dual-isotope approach uses both nitrogen and oxygen isotopes and allows us to distinguish processes that overlap in δ15N alone so that the nitrogen cycle can be disentangled (Casciotti, 2016; Sigman and Fripiat, 2019). In our study region, nitrate stable isotope measurements are still rare. Relatively recently, Bristow et al. (2017) analyzed samples in the central and northern BoB, and Harms et al. (2019) and Marshall et al. (2023) presented nitrate stable isotope data for the subtropical southern Indian Ocean. The East Equatorial Indian Ocean (EEIO) remains largely understudied compared to other major ocean bodies (Ummenhofer and Hood, 2024). Including the EEIO and BoB in our study allows us to compare nitrogen turnover across contrasting oxygen regimes and to assess regional differences in nitrate transformation pathways.
In this study, we use water column properties (temperature, salinity, oxygen, nutrients, and dual stable isotope signatures of nitrate) to study the nitrogen cycle in both the EEIO and the BoB. The major objectives of our study were to (1) determine the isotopic signatures of dissolved nitrate in the water column, (2) decipher the major factors affecting the isotopic signatures of dissolved nitrate, and (3) identify the major nitrogen turnover processes (i.e., nitrate production and consumption) in the EEIO and BoB.
2.1 Study site
The Indian Ocean is unique because the Asian landmass forms a northern boundary in the subtropics, which affects the BoB in many ways. The Asian landmass leads to seasonally reversing monsoon winds in the northern Indian Ocean, which reverse direction from southwesterly winds in summer (June to August) to northeasterly winds in winter (December to February). The upper surface circulation in the BoB and the zonal current system along the Equator are strongly affected by the reversing monsoon system.
In the EEIO (located between 5° S–5° N and 80° E–100° E; Fig. 2), westerly winds emerge during the transition phase in spring (April and May) and again in fall (October and November), leading to strong eastward surface jets, the Wyrtki Jets (Wyrtki, 1973). This leads to annual mean westerly winds that favor downwelling and the absence of a permanent equatorial undercurrent. This downwelling circulation is characterized by the Ekman flow at the surface and divergent geostrophic flow in the thermocline (Phillips et al., 2024).
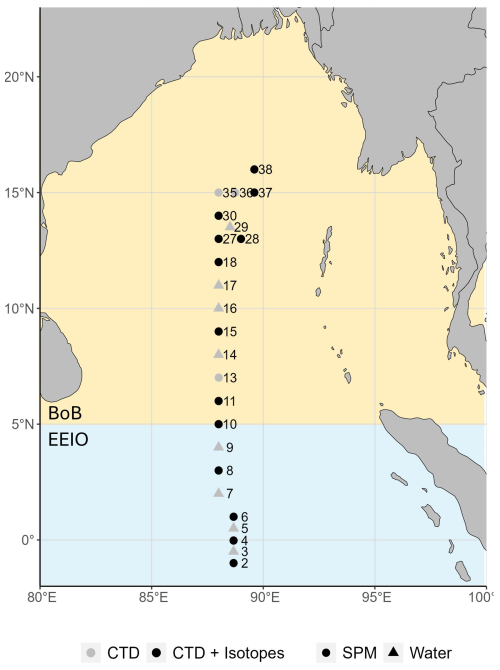
Figure 2Station location sampled in the EEIO and BoB in April and May 2024. The blue background highlights the EEIO (<5° N), and the orange background highlights the BoB (>5° N). Stations shown in gray are where conductivity, temperature, and depth (CTD) and nutrient measurements were conducted. Stations marked in black additionally include nitrate stable isotope sample collection. Suspension samples for particulate nitrogen measurements were done at stations marked with points.
In the BoB, which forms the northeastern basin of the Indian Ocean located between 5–23° N and 80–95° E (Fig. 2), the changing monsoon wind system leads to seasonally reversing ocean surface currents and – because of the monsoonal rainfall – a maximum discharge of river systems draining in the BoB such as the Ganges–Brahmaputra and the Irrawaddy during the summer monsoon (Tomczak and Godfrey, 1994). The large amount of freshwater input from these rivers (1.6×1012m3 yr−1; Sarma et al., 2016) causes a permanent decrease in the salinities in surface waters of the northern and central BoB. High surface temperatures further contribute to large upper-ocean stratification that restricts vertical mixing. This leads to nutrient depletion and thus oligotrophic conditions in surface waters of the BoB (e.g., Kumar et al., 2004; Rao et al., 1994; Sarma, 2002; Shetye, 1993).
2.2 Sampling
During the research cruise SO305 BIOCAT-IIOE2 (10 April–22 May 2024; from Colombo (Sri Lanka) to Singapore), full-depth conductivity, temperature, and depth (CTD) casts were performed at 26 stations on board the R/V Sonne (Fig. 2). The CTD measurements were carried out with a Seabird CTD system that was equipped with a pressure sensor and two parallel sensor sets for conductivity (salinity), temperature, and oxygen. Niskin bottles were attached to the CTD to sample the water column at selected water depths. Seawater samples were collected to calibrate the oxygen and conductivity sensors and to measure nutrient concentrations and nitrate stable isotope composition. For nutrients, a full-water column profile was sampled at every station. Nitrate stable isotope samples were collected at 13 stations, and particulate nitrogen concentrations were collected at 16 stations (Fig. 2).
Oxygen concentrations were measured with SBE 43 Dissolved Oxygen Sensors (Seabird Scientific) and were calibrated with oxygen measurements from discrete water samples, applying the Winkler titration method (Hansen, 1999; Winkler, 1888). For the calibration, a linear correction polynomial depending on pressure, temperature, and the actual oxygen value was fitted. The uncertainty of the CTD oxygen sensor calibration was determined to be an rms of ±0.8 µmol kg−1. However, with the Winkler titration method, oxygen concentrations below about 2 to 3 µmol kg−1 are not detectable, and oxygen concentrations below about 3 µmol kg−1 could not be measured with the CTD oxygen sensor.
Salinity measurements from the Seabird CTD were calibrated against discrete water samples. Sample salinities were measured using Optimare Precision Salinometers, and the calibration was derived following GO-SHIP Repeat Hydrography Manual recommendations (Kawano, 2010), resulting in an accuracy of the calibrated CTD salinities of approximately ±0.002 g kg−1.
2.3 Nutrient analysis
Nutrient concentrations (nitrate, nitrite, silicate, and phosphate) were analyzed on board using a continuous flow analyzer (CFA, SEAL Analytical) using standard colorimetric methods (Hansen and Koroleff, 1999). Dissolved ammonium concentrations were measured with the ortho-phthalaldehyde (OPA) fluorometric method following Holmes et al. (1999). The detection limits were 0.015 µM for , 0.004 µM for , 0.065 µM for , 0.013 µM for , and 0.025 µM for SiO2. We calculated nitrogen deficits (Ndef) according to Bristow et al. (2017):
where is the sum of nitrate and nitrite concentrations; is the average ratio between to phosphate () in the deepest water samples of each station, ranging between 2500 to 4500 m, with this ratio amounting to 14.16 during the cruise; and is the phosphate concentration.
Particulate nitrogen sampling was conducted at five to nine different water depths at each suspension station (Fig. 2). Approximately 30 L of seawater was filtered on pre-combusted (450 °C, overnight) Whatman GF/F filters and dried at 40 °C for 48 h. Dried filters were used to the determine the suspended particulate matter and particulate nitrogen concentrations. For the measurements of particulate nitrogen, a laboratory hole puncher was used to extract defined pieces from the filter (punch area: 20.43 mm2). These were measured using an Elemental Analyzer (Euro Vector EA 3000) calibrated against a certified acetanilide standard (IVA Analysentechnik, Germany). The standard deviation was 0.005 % for nitrogen.
2.4 Nitrate stable isotope analysis
For the determination of nitrate stable isotope signatures, water samples were filtered (GF+SFCA, MINISART, 0.45 µm) on board and stored frozen (−20 °C) in Falcon PE (polyethylene) tubes until analysis in the home lab. The samples were shipped as frozen airfreight to Germany. stable isotopes were measured using the denitrifier method (Casciotti et al., 2002; Sigman et al., 2001). in the filtered water sample was reduced to nitrous oxide by Pseudomonas aureofaciens (ATCC#13985). The produced nitrous oxide was analyzed by a GasBench II coupled with an isotope ratio mass spectrometer (Delta Plus XP, Thermo Fisher Scientific). For calibration, two international standards (USGS34: δ15N– ‰ and δ18O– ‰; IAEA: δ15N– ‰ and δ18O– ‰) and one internal standard (δ15N– ‰ and δ18O– ‰) were measured in each run. The standard deviation for standards and samples was usually <0.2 ‰ for δ15N– and <0.5 ‰ for δ18O–. Nitrite was usually below the detection limit, leading to the assumption that isotopes are representative for isotopes. If the nitrite concentration exceeded 5 %, it was removed prior to analysis using sulfamic acid (Granger and Sigman, 2009) to get nitrate isotope signatures.
Nitrite can significantly influence the measured δ18O–, even if nitrite is present in seawater at very low concentrations. During bacterial conversion to N2O, nitrite undergoes a smaller fractional loss of oxygen atoms compared to nitrate, leading to lower oxygen isotopic fractionation during nitrite reduction to N2O (about 25 ‰ less) than when N2O is generated from nitrate with the same initial δ18O (Casciotti and McIlvin, 2007). As a result, calibrating measured oxygen isotope ratios to nitrate reference materials leads to an underestimation of the δ18O of . To account for this methodological bias, we corrected the δ18O of by using the measured nitrite concentration relative to that of for each sample. The δ18O data for presented these corrected values (Fawcett et al., 2015; Peng et al., 2018).
In the deep ocean, nitrate is produced through nitrification, which utilizes ambient water as the main source of oxygen atoms (Buchwald et al., 2012; Casciotti et al., 2002, 2010). Consequently, changes in the δ18O of seawater are reflected in the δ18O of the produced nitrate. To account for this, we corrected the δ18O for the salinity-driven depth variations in seawater δ18O (δ18ONO3, sal corrected) following the method outlined by Knapp et al. (2008):
where δ18ONO3 denotes the measured values with nitrite correction, sal is the salinity of the sample, and sal1000 m is the salinity at 1000 m depth at each station. The factor 0.16 is the approximate slope between seawater δ18O and salinity for the Indian Ocean (LeGrande and Schmidt, 2006).
We calculated nitrate isotopic anomalies Δ(15,18) according to Sigman et al. (2005):
where δ15N and δ18O are nitrate isotopes of the sample, and δ15Ndeep and δ18Odeep are the deep-water values for each station. The isotopic effects (ε) for denitrification are equal so that equals 1 if no other process is involved.
The δ15N of nitrite was calculated via an isotopic mass balance:
where , , and are the nitrate isotopes of nitrite, the sum of nitrite and nitrate, and nitrate, respectively.
3.1 Nutrient distribution
Surface waters (<50 m) were depleted in nutrients, with nitrate often being below the detection limit (Fig. 3a and k), leading to an average nitrate concentration of 0.7±2.3 µmol L−1. With depth, nitrate concentrations increased, with the highest concentrations at ∼900 m (maximum values of 38.5 µmol L−1 at station 28). With further depth, these decreased to average bottom water concentrations of 34.9±0.7 µmol L−1.
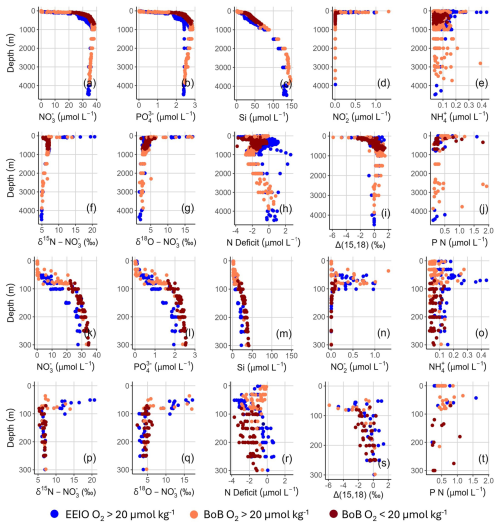
Figure 3Depth profiles for concentration of nitrate for (a) 0–4500 m and (k) 0–300 m, concentration of phosphate for (b) 0–4500 m and (l) 0–300 m, concentration of silicate for (c) 0–4500 m and (m) 0–300 m, concentration of nitrite for (d) 0–4500 m and (n) 0–300 m, concentration of ammonium for (e) 0–4500 m and (o) 0–300 m, δ15N– for (f) 0–4500 m and (p) 0–300 m, δ18O– for (g) 0–4500 m and (q) 0–300 m, Ndef for (h) 0–4500 m and (r) 0–300 m, Δ(15,18) anomalies for (i) 0–4500 m and (s) 0–300 m, and particulate nitrogen concentrations for (j) 0–4500 m and (t) 0–300 m. Colors indicate regions (blue points for the EEIO <5° N and orange points for the BoB >5° N; see Fig. 2). Samples within the BoB with oxygen concentrations <20 µmol kg−1 are displayed as dark-red points.
Phosphate concentration followed a similar pattern, with the lowest values in surface waters, increasing to a maximum of 2.9 µmol L−1 at 900 m (station 28). In bottom waters, phosphate levels declined slightly to an average of 2.5±0.1 µmol L−1 (Fig. 3b and l). Silicate concentrations increased with depth, starting from low surface values and reaching average bottom water concentrations of 133.7±20.6 µmol L−1 (Fig. 3c and m).
A small nitrite maximum existed at ∼70 m, with maximum values of >1 µmol L−1 (Fig. 3d and n). At the same depth, a small increase in ammonium concentrations was measured up to 0.4 µmol L−1 (Fig. 3e and o). Otherwise, nitrite and ammonium were low and often below the detection limit.
The calculated Ndef was highest in the upper 300 m, peaking at −3.7 µmol L−1 at 111 m at station 36. Below ∼100 m, the Ndef was reduced to ∼350 m, reaching a maximum nitrogen excess of +1 µmol L−1. Below 350 m, the Ndef increased again down to 1000 m before decreasing once more to approach ∼0 µmol L−1 in the deeper water layers (Fig. 3h and r). It is striking that station 2 (1° S and 88.40° E) exhibited NOx excess up to 2.6 µmol L−1 at 1000 m depth, whereas all of the other stations showed a deficit at the same depths (Fig. 3h). This anomaly seems to be primarily driven by unusually low phosphate concentrations between 500 and 3000 m at station 2 (Fig. 3b), while nitrate profiles remain largely consistent with those at adjacent stations (Fig. 3a). Although hydrological boundaries and biological turnover are unlikely explanations and although no clear evidence of measurement errors was found, the cause of this deviation remains unclear.
Particulate nitrogen concentrations were low, ranging between 0.1 and 1.9 µmol L−1, with the highest concentrations in the upper 100 m of the water column (Fig. 3j and t). High particulate nitrogen concentrations in deep water were measured at station 37 at 2573 and 2678 m, with 1.8 and 1.9 µmol L−1, respectively, as well as at station 35 at 340 m, with 1.8 µmol L−1.
3.2 Nitrate stable isotope distribution
The nitrate stable isotopes showed distinct depth profiles (Fig. 3f and g). The heaviest isotope signatures were measured in the shallowest samples, reaching values up to 20.7 ‰ for δ15N– and 17.2 ‰ for δ18O– (Fig. 3p and q). With increasing water depths, isotopic values decreased to 5.3 ‰ at 80 m depth (σθ=23.5 kg m−3, station 18) and 2.6 ‰ at 150 m depth (σθ=25.4 kg m−3, station 4) for δ15N– and δ18O–, respectively. From 150 to 200 m, nitrate isotopes increased. Below 200 m (σθ∼27.5 kg m−3), δ15N– showed little variability, averaging 6.7±0.3 ‰. Below 1000 m, δ15N– declined, approaching average deep-water values of 5 ‰ (Sigman et al., 2000). δ18O– displayed greater variability, especially in more shallow samples, with notably enriched δ18O– values observed towards the north. The greatest spatial variation occurred at approximately 150 m water depth, with samples collected in the south being depleted in δ18O– and samples in the north being enriched in δ18O–, ranging from 2.6 ‰ at station 4‰ to 6.2‰ at station 38. In deep waters, δ18O– approached 5 ‰.
The calculated Δ(15,18) anomalies were negative in shallow waters (>2 ‰). The anomalies increased with water depth, leading to positive values at depths of 300 to 3000 m and approaching 0 ‰ in deep waters (Fig. 3i and s).
3.3 Physical water column properties
The thermocline waters along the ∼88° E section showed a strong separation of the water properties of salinity and oxygen (Fig. 4a and b). North of 5° N, the upper 80 m were characterized by low salinities of <34 and high temperatures of >27 °C. South of 5° N, a salinity maximum of >35.3 was observed below the surface layer (σθ>22.7 kg m−3) at water depths of 70 to 120 m and was carried northwards up to ∼5° N. In deeper water layers (water depth ∼100 to 580 m, σθ∼24.5 to 27.1 kg m−3), we observed decreasing salinities in the south and increasing salinities in the north. At ∼580 m (σθ∼27.1 kg m−3), salinities in both regions were comparable (∼35), followed by a small decrease in deeper waters (Fig. 4a and c).
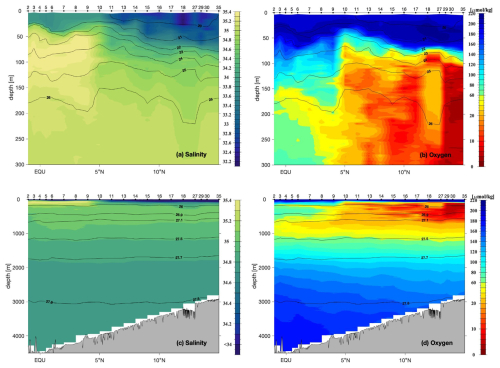
Figure 4Vertical distribution of (a, c) salinity and (b, d) oxygen (in µmol kg−1) in the upper 300 m depth (a, b) and in the entire water column along the ∼88° E–88.7° E section from CTD measurements during SO305 in April and May 2024. Potential density surfaces are contoured in black. Black dots and numbers denote the ship station numbers (also shown in Fig. 2).
The surface water (<90 m) was saturated in oxygen, and oxygen concentrations decreased with water depth. North of 5° N, an oxygen-minimum zone existed at 100 to 750 m, with oxygen concentrations <20 µmol kg−1, reaching minimum values below the detection limit of 3 µmol kg−1 at 120 to 220 m water depth. In the south, oxygen concentrations decreased to ∼50 µmol kg−1. At ∼400 m (σθ∼26.9 kg m−3), an oxygen peak was measured from 1° S to 6° N, followed by a low-oxygen water mass below 580 m (σθ∼27.1 kg m−3). In deep waters, oxygen concentrations increased again to 150 µmol kg−1 (Fig. 4b and d).
3.4 Water mass distribution
Water masses were characterized by their potential temperature (T), salinity (S), density, and oxygen concentration profiles. The T–S diagram (potential temperature versus salinity) for this study allowed us to identify two different, clearly separated, identifiable water mass distributions within the study area (Fig. 5a): the EEIO south of 5° N and the BoB north of 5° N. The T–S profiles diverge into two distinct branches in the mixed layer and in the thermocline below σθ values of approximately 27 kg m−3, with higher salinity water masses observed in the EEIO and low-salinity water masses found in the BoB (Fig. 5a). The same separation was identified in the σθ vs. salinity (Fig. 5b) and σθ vs. oxygen plots (Fig. 5c). Only one station at 5° N showed mixing between water masses of the BoB and EEIO (Fig. 5a and b), indicating that the exchange of water masses between the BoB and southern hemisphere is limited due to the blocking effect of the equatorial current system (Schott and McCreary, 2001).
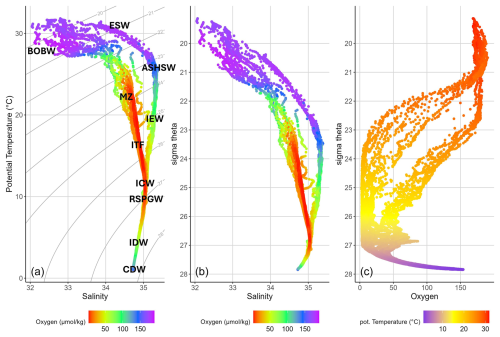
Figure 5(a) T–S (potential temperature versus salinity) diagram from CTD measurements with marked water masses. The color bar indicates oxygen concentrations (in µmol kg−1), and gray lines indicate density surfaces in σθ (in kg m−3). (b) σθ (in kg m−3) plotted against the salinity diagram, with the color bar indicating oxygen concentrations (in µmol kg−1). (c) Sigma-theta (in kg m−3) plotted against oxygen (in µmol kg−1), with color bar indicating potential temperature in °C. Abbreviations: Bay of Bengal Water – BoBW, equatorial surface water – ESW, Arabian Sea High-Salinity water – ASHSW, mixed zone – MZ, Indian Equatorial Water – IEW, Indonesian Throughflow Water – ITF, Indian Central Water – ICW, Red Sea Persian Gulf Water – RSPGW, Indian Deep Water – IDW, and Circumpolar Deep Water – CDW.
In the Bay of Bengal (>5° N), T–S diagrams were largely consistent with those of previous studies (Fig. 5a; Emery, 2001; Rahman et al., 2021; Sengupta et al., 2013; Tomczak and Godfrey, 1994), which identified the following water masses in the BoB: (1) the Bay of Bengal Water (BoBW), (2) the mixed zone (MZ), (3) the Indonesian Throughflow (ITF), (4) Indian Central Water (ICW), (5) Indian Deep Water (IDW), and (6) Circumpolar Deep Water (CDW).
The BoBW in the near-surface water of the BoB is characterized by extremely low salinities (S<33.5) due to the large amount of freshwater input through the combination of strong river discharge and excess precipitation (Schott and McCreary, 2001). Furthermore, other studies detected Arabian Sea High-Salinity Water (ASHSW) and Red Sea Persian Gulf Water (RSPGW) in the BoB (Jain et al., 2017; Rahman et al., 2021; Sheehan et al., 2020) (Fig. 5a). The ASHSW spreads eastward in the upper 100 m depth with the Southwest Monsoon Current (SMC) around the southern tip of Sri Lanka during summer and northward into the BoB with the East India Coastal Current (EICC) and slides below the BoBW (Vinayachandran et al., 1999). Our measurements in the BoB did not show the presence of ASHSW north of 5° N, possibly because our cruise took place during the spring transition phase. This aligns with Jain et al. (2017), who found that ASHSW disappears to the east and north of the south–central bay (8° N, 85° E) as a result of mixing with the BoB's fresher surface waters. Maximum salinity across most of the BoB is attributed to Red Sea Persian Gulf Water (RSPGW). There is still discussion about the extension of Persian Gulf Water and Red Sea Water into the BoB. Nonetheless, the presence of RSW and PGW in the BoB has been previously shown by Jain et al. (2017) and Sheehan et al. (2020) (Fig. 5a).
The BoB thermocline waters of the upper 1000 m depth mainly originate from the Southern Hemisphere due to the geographical boundary in the north. The thermocline waters primarily consist of ICW and water originating from the ITF (You and Tomczak, 1993). The ITF brings relatively low-salinity water (S<34.6) originating from the tropical Pacific Ocean and merges with the South Equatorial Current (SEC) on its way to the western boundary (Gordon, 2005; Sprintall et al., 2009; You, 1997). ICW is formed along the subtropical convergence zone by subduction and is carried westward by the SEC (Sprintall and Tomczak, 1993; You, 1997). ICW is characterized by higher-salinity waters (S>35, Fig. 5a) and a quasi-linear relation above 7 °C (Schott and McCreary, 2001; Sverdrup et al., 1942). During the summer monsoon, both water masses cross the Equator and flow into the Arabian Sea and eventually into the BoB (Schott et al., 2009; Tomczak and Godfrey, 1994; You, 1997).
In the thermocline waters of the EEIO, we identified the presence of equatorial surface water (ESW), ASHSW, Indian Equatorial Water (IEW), and RSPGW. The IEW mainly consists of ITF, with contributions from the ICW (Tomczak and Godfrey, 1994). The observed separation between the BoBW and ESW at 5° N aligns with findings by Carvalho Junior (2023), who identified ESW between 10° S and 5° N, extending westwards from 60° E. Shee et al. (2023) assigned the most upper 50 m with low salinities to the BoBW, with ESW being present from 50 to 100 m. However, in our observation, the pronounced salinity maximum between σθ 23 and 24 kg m−3 shows the presence of ASHSW at water depths between 35–90 m, contrasting with the absence of this water mass in the BoB (Fig. 5a).
IDW originates from North Atlantic Deep Water, which is transported into the Indian Ocean with the Circumpolar Current and spreads northward along the western boundary (Tomczak and Godfrey, 1994). IDW can be found below about σθ=27.6 µmol kg−1, with oxygen values between 100 and 200 µmol kg−1 ventilating the thermocline from below (Ditkovsky et al., 2023). CDW can be found just below the IDW, with a salinity of about S=34.7 and very low temperature (Schott and McCreary, 2001).
3.5 Correlation analysis
We examined the relationship of nitrate isotopes with salinity, potential temperature, and multiple-regression analysis of both isotopes using salinity and potential temperature (see the Supplement). A strong correlation of isotope changes with water mass tracers generally suggests that water masses are the main drivers of change, whereas a lack of correlation implies additional processes. Isotopic variability showed no strong correlation in either the BoB or EEIO when data from all depths were analyzed (best fit with multiple-regression analysis: R2<0.24 and p<0.001 and R2<0.58 and p<0.001 for δ15N– and δ18O–NO3, respectively, in the BoB and R2<0.47 and p<0.001 and R2<0.52 and p<0.001 for δ15N– and δ18O– in the EEIO). This indicates that water mass changes are not the sole driver for isotopic variability within the entire water column. The correlations improved significantly after excluding the upper water masses from both regions. In the BoB, removing the measurements done in the BoBW and MZ from the dataset (water depth from 0 to 170 m) led to strong significant correlations for both (best fit with multiple-regression analysis: R2=0.84, p<0.001) and δ18O– (best fit with potential temperature: R2=0.65, p<0.001), which was further improved by excluding measurements from the ITF (best fit with multiple-regression analysis: depths to 300 m, R2=0.89 and p<0.001 and R2=0.61 and p<0.001 for δ15N– and δ18O–, respectively). In the EEIO, excluding measurements from the ESW, ASHSW, and IEW (0–225 m water depth) led to strong significant correlations (best fit with multiple-regression analysis: R2=0.84 and p<0.001 and R2=0.55 and p<0.001 for δ15N– and δ18O–, respectively).
4.1 Correlation analysis
The nitrate stable isotopes exhibited distinct depth profiles, with significant variation in the upper 300 m and minimal variation at greater depths. In the deepest samples, δ15N– declines, approaching average deep-water values of 5 ‰ (Sigman et al., 2000). These isotopic changes can result from changes in in situ nitrogen turnover processes, as well as changes in water masses. For the latter case, nitrogen concentration and isotope signatures are not due to processing in the BoB but are inherited from the region of water mass formation and are transported to the study site (e.g., Harms et al., 2019; Marshall et al., 2023).
To evaluate the extent to which variations in nitrate stable isotopes can be attributed to changes in water mass contribution, we examined the relationship of nitrate isotopes with salinity and potential temperature (Sect. 3.5), showing that water masses are not the sole driver for isotopic variability, with no strong correlation in either the BoB or EEIO when data from all depths were analyzed. Excluding water masses in the upper 200 to 300 m of the water column improved the correlations significantly, leading to strong significant correlations of water mass tracers with dual nitrate stable isotopes. Therefore, we conclude that the δ15N– and δ18O– in the deeper water were water mass specific and reflect variations in the water mass distributions, while the nitrate isotopic signatures in the upper 300 m were influenced by in situ nitrate fractionation.
4.2 Nitrate turnover in the upper 300 m of the water column
In the upper 300 m, nitrate isotope depth profiles showed significant variation, with the highest values in the uppermost samples, the lowest values around 70–90 m (σθ∼22–24 kg m−3), and a slight increase with greater depth (Fig. 3). A comparison of nitrate stable isotopes in the upper water column between the EEIO and BOB reveals several notable differences: (1) similar δ15N– depth profiles in the EEIO and BOB with distinct variabilities (Fig. 3p); (2) enriched δ18O– in the BoB with oxygen concentrations below 20 µmol kg−1 compared to lower values in the oxic EEIO (Figs. 3q and 6c); and (3) persistent Ndef between 100 and 300 m (σθ: 24–27 kg m−3) in the BoB (Fig. 3r), while, in the EEIO, the Ndef is markedly reduced below ∼100 m. Although some EEIO samples do show Ndef at depths >100 m, these are significantly less pronounced than in the BoB and likely reflect an source due to coupled remineralization and nitrification processes counterbalancing the Ndef. We also acknowledge a potential bias in our Ndef calculation (Eq. 1) based on deep-water samples at each station. Applying this deep-water ratio to shallower layers may overestimate the Ndef in the upper water column, especially if the ratio varies with depth due to biological or physical processes.
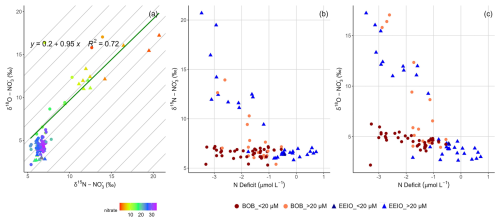
Figure 6Nitrate isotope signatures and nitrate deficit for samples in the upper 300 m: (a) δ18O– plotted against δ15N–. Regression lines, equations, and R2 values were only calculated for surface water samples (<100 m). Diagonal gray lines have a slope of 1. Colors indicate nitrate concentrations, and shapes indicate the region of the sample (filled triangles stand for the EEIO, and full circles stand for the BoB). (b) δ15N– and (c) δ18O– plotted against Ndef. Colors and shapes indicate the region (blue-filled triangles stand for the EEIO <5° N, and orange-filled circles stand for the BoB >5° N). Filled dark-red circles display samples in the BoB with oxygen concentrations <20 µmol kg−1.
Taken together, the vertical and regional patterns in nitrate isotopes and Ndef suggest distinct nitrate cycling in the BoB and EEIO. The isotopic variations with depth suggest changes in nitrate cycling with depth, while the regional decoupling between δ15N– and δ18O– shows regional differences in nitrate cycling in the EEIO and BoB.
4.2.1 Surface waters
In the majority of the samples from water depths above ∼80 m, δ15N– and δ18O– increased significantly up to >20 ‰ and >15 ‰, respectively (Fig. 3p and q). We attribute this simultaneous increase in both isotopes, along with low nitrate concentrations (Fig. 3k, p, and q), in oxic surface waters to nitrate assimilation. Upper-ocean assimilation has been observed and documented in numerous studies (e.g., Fawcett et al., 2015; Peng et al., 2018; Wankel et al., 2007). During nitrate assimilation, phytoplankton preferentially utilize the lighter isotopes, resulting in an enrichment of the remaining nitrate pool as its concentration decreases (Fig. 1). Culture studies showed that nitrate assimilations lead to a ratio of δ18O to δ15N of 0.96–1.09 (Granger et al., 2004, 2010; Karsh et al., 2012), which matches the ratio of 0.95 (R2=0.70, p<0.01) found in our study (Fig. 6a). This indicates active phytoplankton uptake as the dominant nitrate sink in the surface water of the BoB and EEIO (Fig. 7).
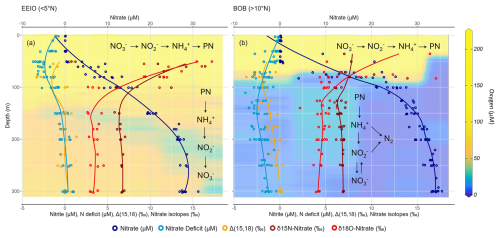
Figure 7Schematic overview of nitrogen turnover processes in the (a) EEIO and (b) BoB in the upper 300 m of the water column. The figure shows individual measurements and moving averages for nitrate (µmol L−1), Ndef (µmol L−1), Δ(15,18) (‰), δ15N– (‰), and δ18O– (‰) for each region. Nitrate is shown in dark blue, Ndef is shown in light blue, δ15N– is shown in dark red, δ18O– is shown in light red, and Δ(15,18) is shown in orange. The background indicates measured oxygen concentrations (µmol kg−1). The zone of nitrate assimilation in surface waters is evidenced by high δ15N– and δ18O– values, as well as low nitrate concentrations and an Ndef. In the EEIO, subsurface water are dominated by coupled remineralization and nitrification, which shows a reduced Ndef and lighter nitrate isotopes. In the BoB, subsurface waters with low oxygen concentrations also show signs of nitrate reduction by a persistent Ndef below 100 m, along with enriched δ18O– values that lead to negative Δ(15, 18) anomalies. This can be explained by nitrate reduction, which might be fueled by nitrate production during nitrification, coupled with anammox.
In the warm, sunlit ocean, N2 fixation may produce newly bioavailable nitrogen (Fig. 1, Marshall et al., 2023). However, in the BoB, Löscher et al. (2020) found no consistent evidence for N2 fixation, whereas Saxena et al. (2020) detected N2 fixation but assessed its contribution to primary production to be negligible. Shiozaki et al. (2014) only found low N2 fixation rates in the southern and Equatorial Indian Ocean, with similar minimal influence on primary production (Saxena et al., 2020). Since δ15N– is not significantly more depleted than δ18O–, which would be typical for N2 fixation (Marshall et al., 2023), and in agreement with previous studies (Löscher et al., 2020; Saxena et al., 2020; Shiozaki et al., 2014), N2 fixation seems to be negligible in the BoB and EEIO in our study.
In surface waters, another possible nitrogen source is atmospheric deposition that is reflected in low δ15N– (−14 ‰ to 2 ‰; Altieri et al., 2021) and high δ18O– (>60 ‰; Kendall et al., 2007) values. Thus, the observed simultaneous increase in both nitrate isotopes contradicts significant nitrate input by atmospheric aerosol deposition, which is in line with the findings of Sarma (2022). SO305 took place during the transition period and the early stage of the southwestern monsoon. The prevailing southwesterly winds were, therefore, not favorable for a pronounced aerosol transport from the land and led to low aerosol deposition at the time of our study (Bange et al., 2024).
4.2.2 Subsurface waters
At ∼70–90 m (σθ: 22–24 kg m−3), a nitrite maximum exists, with concentrations of >1 µmol L−1 (Fig. 3n). Just below the nitrite maximum, the lowest nitrate isotopes were measured (Fig. 3p and q) alongside a steep increase in nitrate concentration (Fig. 3k) and reduced Ndef (Fig. 3r), indicating nitrate production via nitrification. Ammonium was most probably produced via remineralization of organic material originating from either new production or regenerated production (Fig. 1; Marshall et al., 2023), even though rapid nitrification can disguise this production by immediate oxidation of freshly produced ammonium.
Our data (Fig. 6a) are consistent with findings from the Sargasso Sea, where Fawcett et al. (2015) observed changes in δ18O– without corresponding shifts in δ15N–, indicating nitrification alongside partial nitrate assimilation. The decoupling occurs because nitrification produces nitrate with relatively high δ18O values, while its δ15N remains like that of organic nitrogen remineralized. At the same time, nitrate assimilation removes lower δ18O, causing the δ18O of the remaining nitrate to increase more than δ15N. Additionally, ammonium in the euphotic zone is more likely to be oxidized than assimilated due to a large isotope effect during ammonium oxidation. This introduces low δ15N–, further reducing δ15N relative to δ18O (Fawcett et al., 2015).
The formation of the primary nitrite maximum (PNM) at the base of the euphotic zone may result from an imbalance between ammonium oxidation and nitrite oxidation when ammonium oxidation is higher than nitrite oxidation. We calculated δ15N– via a mass balance. All calculated values were extremely low, with an average of −58.1 ‰. Due to the low concentration, the calculation of nitrite isotopes is prone to errors. However, values below −14 ‰, as in this case, indicate ongoing nitrite oxidation, which exhibits an inverse kinetic isotope effect (−9–−20 ‰; Buchwald and Casciotti, 2010; Casciotti, 2009) and amplifies the 15N depletion of nitrite caused by nitrate reduction (Fig. 1; Casciotti, 2009).
The observed δ18O– minimum values of 2.6 ‰ and 3.6 ‰ in the EEIO and BoB, respectively, are close to the global mean oceanic value of 2.4 ‰ relative to the nitrification source (Sigman et al., 2009), indicating freshly nitrified nitrate.
Since we did not find any indication of significant N2 fixation (Sect. 4.2.1), it appears to be the case that primarily regenerative nitrate production by nitrification of phytoplankton organic matter takes place in the BoB and EEIO (Fig. 7).
4.2.3 Nitrogen deficit in subsurface waters of the BoB
Between 100 and 300 m, the BoB exhibited a significant Ndef, reaching up to −3.7 µmol L−1 (Fig. 3r). Oxygen concentrations in the BoB at depth of the Ndef were low and fell below 20 µM, and, in some cases, oxygen concentrations even dropped below the detection limit of 3 µM (Fig. 4b). Such low oxygen concentrations are theoretically favorable for nitrate removal via anammox or denitrification (Bristow et al., 2016; Dalsgaard et al., 2014; Kalvelage et al., 2011; Rixen et al., 2020).
Generally, water column nitrate removal should be accompanied by an isotopic enrichment of nitrate. In the adjacent OMZ of the Arabian Sea, such removal processes result in substantial enrichment of nitrate stable isotopes, with values reaching up to 30 ‰ for both δ15N– and δ18O– (Gaye et al., 2013; Martin and Casciotti, 2017; Naqvi et al., 1998). Although the isotopic enrichment observed in the BoB is less pronounced, we still detect a significant signal: samples characterized by persistent Ndef and low oxygen concentrations were accompanied by a small but notable enrichment of both δ15N– and δ18O– (Fig. 6b and c). This enrichment clearly differs from nitrate dual-isotope signatures in the oxic water column in the BoB and EEIO (Fig. 3e and g), providing strong evidence that nitrogen removal processes are indeed active in the BoB.
Within the OMZ of the BoB, nitrate stable isotopes reached values up to 7.2 ‰ and 6.3 ‰ for δ15N– and δ18O–, respectively. This corresponds to an enrichment of 1.7 ‰ and 4.1 ‰, respectively, over deep-water values. The enhanced enrichment of δ18O– in the BoB becomes particularly evident in comparison with the EEIO, where δ18O– values were notably lighter (Fig. 3q). Theoretically, a shift in δ18O−H2O can lead to changes in δ18O– of freshly produced nitrate because oxygen from water is incorporated (Kool et al., 2007; Snider et al., 2010). However, the BoB and EEIO have similar δ18O−H2O signatures (Kim et al., 2021; Sengupta et al., 2013; Srivastava et al., 2007). Therefore, we argue that the observed δ18O– shift and enrichment in the BoB was not caused by regional δ18O−H2O differences but is rather caused by differences in nitrate turnover between the BoB and EEIO. Based on the correlation of δ18O– increases with Ndef in the BoB (Fig. 6c), we conclude that the main differences are linked to nitrate loss in the anoxic water column of the BoB.
We calculated negative Δ(15,18) anomalies ranging from −2 ‰ to 0 ‰ (Fig. 3s). An enhanced enrichment of δ18O– has been observed before in the BoB (Bristow et al., 2017) and in other OMZs (Gaye et al., 2013; Martin and Casciotti, 2017) and was attributed to coupled anaerobic and aerobic processes involving nitrate reduction to nitrite followed by its reoxidation to nitrate. Studies have shown that, during nitrate reduction, the ratio of the kinetic isotope effect is 1:1 (15ε :18ε) (Granger et al., 2008). However, repeated nitrate reduction and nitrite oxidation cause few changes in the isotopic signature of δ15N– but lead to an increase in δ18O– values because nitrite oxidation fractionates strongly on nitrogen, with −9 ‰ to −20 ‰, and less on oxygen (−1 ‰ to −8 ‰, respectively) (Buchwald and Casciotti, 2010; Casciotti, 2009). The nitrate that undergoes reduction initially has a lower δ18O value than the reoxidized nitrite, causing the negative Δ(15,18) anomaly (Gaye et al., 2013). Thus, in the OMZ of the BoB, the combination of an Ndef with observed isotopic enrichment might result from ongoing nitrate reduction–reoxidation cycles and partial nitrite consumption of nitrite by anammox (Fig. 7).
Bristow et al. (2017) identified a significant potential for nitrogen removal via anammox, with little evidence of denitrification in the BoB. Based on their measurement of oxygen at the trace level, they hypothesized that these trace oxygen levels enable nitrite-oxidizing bacteria to outcompete anammox bacteria for available nitrite, leading to nitrite limitation of anammox rather than ammonium or oxygen limitation. Anammox bacteria are known for their tolerance of ambient oxygen levels, with tolerances as high as 20 µM (Okabe et al., 2023), making their contribution to the Ndef more likely than denitrification. Denitrification, shown to be driven by episodic supply of organic matter (Ward, 2013), might also be limited by low particulate nitrogen availability in the BoB (Fig. 3j and t). Furthermore, Bristow et al. (2017) found similar isotopic enrichments in nitrate isotopes and negative Δ(15,18) anomalies that match our data. Thus, anammox appears to be the more plausible nitrogen loss pathway in the BoB. Nitrogen removal by anammox can explain the observed nitrogen deficit, as well as the dual-isotope enrichment of nitrate.
Intriguingly, anammox bacteria can also produce nitrate via reoxidation of nitrite as they fix carbon dioxide into biomass using reducing equivalents produced during the oxidation of nitrite into nitrate (Dalsgaard et al., 2003; Kobayashi et al., 2019). The nitrate production via anammox bacteria has a significant impact on nitrite and nitrate dual isotopes but is still often overlooked (Granger and Wankel, 2016; Kobayashi et al., 2019). Similarly to conventional nitrification, nitrite oxidation via anammox has an inverse isotopic effect (Brunner et al., 2013; Kobayashi et al., 2019), with a significant higher isotope effect in 15ε, ranging from −30 ‰ to −45 ‰, compared to that in 18ε, ranging from −2 ‰ to −12 ‰ (Kobayashi et al., 2019). Thus, nitrite oxidation by anammox bacteria could contribute to the decoupling of nitrate isotopes and the resulting enrichment of δ18O–, further supporting anammox as the most important nitrogen loss pathway in the OMZ of the BoB.
This study sheds light on the nitrogen cycle in the EEIO and BoB. We used nitrate stable isotope signatures to disentangle turnover processes and reveal fundamental regional differences in nitrogen cycling between both regions. A detailed analysis of water mass distributions in both regions revealed that the OMZ in the BoB was well separated from the EEIO at 5° N. This separation was also reflected in the water column distribution of nitrate isotope signatures. Nitrate isotope signatures in deeper water masses (>300 m) were solely controlled by changes in water mass distribution, whereas, in the upper 300 m, isotope variations were caused by on-site nitrate fractionation. In surface waters (0–80 m), one active nitrate sink was phytoplankton assimilation, causing a significant isotopic enrichment and Ndef in the upper 50 m. In the underlying waters, nitrification took place, most presumably fueled by regenerated phytoplankton biomass rather than by remineralization of nitrogen that was freshly fixed by N2 fixation. In the OMZ of the BoB between 100 and 300 m, we found a persistent Ndef, along with nitrate isotope signatures that were slightly but significantly enriched compared to deep-water values and samples from the same depths in the EEIO. Given the dynamic oxygen concentrations in the water column (Bristow et al., 2017; Johnson et al., 2019), anammox appeared to be the more plausible nitrogen loss pathway in the BoB. Overall, we find that there is convincing evidence for nitrate reduction processes in the BoB. To fully assess the significance of nitrogen loss in the Bay of Bengal and the potential future evolution of nitrate reduction pathways, it is essential to conduct detailed studies on nitrogen-cycling processes, measure the rates of potential nitrogen loss mechanism, and explore the microbial communities that drive these transformations within these dynamic and ecologically significant oxygen-minimum zones.
The nitrate isotope data set (https://doi.org/10.1594/PANGAEA.983257, Schulz and Penopp, 2025), are available on PANGAEA (Felden et al., 2023).
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-5943-2025-supplement.
GS and BG designed this study. HWB was the principal investigator during SO305 BIOCAT-IIOE2. GS took the nitrate stable isotopes samples, and RC was responsible for the CTD operations. JP and GS measured the nitrate stable isotope samples. KD, TS, JP, HWB, RC, and BG contributed with scientific and editorial recommendations. GS wrote the paper and prepared the submitted paper with contributions from all of the co-authors.
At least one of the (co-)authors is a member of the editorial board of Biogeosciences. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank the captain and crew of the R/V SONNE for their support during SO305 BIOCAT-IIOE2; Niko Lahajnar for his support with the cruise logistics; Tjark Andersch and Leandro Nazzari for their support during the cruise; Ramazan Cetin for measuring the particulate nitrogen content; Kira Lange, Leon Schmidt, and Jannis Usinger for measuring the nutrient and oxygen concentrations; and the entire CTD team for operating the CTD and water samples on board. We also highly appreciate the scientific feedback we received from Bo Thamdrup and Victor Fernandez Juarez.
Data were visualized by R, specifically ggplot2 (version 3.4.1; Wickham et al., 2016) and ggpubr (version 0.6.0; Kassambara, 2023). The map of our study area was plotted using rnaturalearth (version 1.0.1; Massicotte and South, 2025), rnaturalearthdata (version 1.0.0; South et al., 2025), and sf (version 1.0-18; Pebesma and Bivand, 2023). Correlation analyses were performed with stats (version 4.0.2; R Core Team, 2022).
This article is dedicated to the memory of our colleague and friend, Gerd Krahmann.
This research has been supported by the Bundesministerium für Bildung and Forschung (grant nos. 03G0305A and 03G0305B).
The article processing charges for this open-access publication were covered by the Helmholtz-Zentrum Hereon.
This paper was edited by Tina Treude and reviewed by two anonymous referees.
Altieri, K. E., Fawcett, S. E., and Hastings, M. G.: Reactive Nitrogen Cycling in the Atmosphere and Ocean, Annu. Rev. Earth Pl. Sc., 49, 523–550, https://doi.org/10.1146/annurev-earth-083120-052147, 2021.
Bange, H. W., Stoltenberg, I., Andersch, T., Arévalo-Martínez, D. L., Atlas, E. L., Babu Suja, A., Barbot, A., Barthelmeß, T., Becker, K. W., Booge, D., Bristow, L. A., Conventz, A., Czeschel, R., Dähnke, K., Deshmukh, S., Duerkop, F., Eisnecker, P., Engel, A., Engelen, B., Feil, H., Fernández-Juárez, V., Firus, A., Frank, M., Gaye, B., Gledhill, M., Golde, S., Großelindemann, H., Hathorne, E., Henning, S., Hentschel, I., Herrmann, H., Ingeniero, R. C. O., Jacobsen, M., Kiko, R., Lahajnar, N., Lange, K., Löscher, C. R., Marandino, C. A., McKellar, C., Mickenbecker, J., Müller, M., Müller, T., Nazzari, L., Nielsen, L., Ploschke, J., Pohlker, M., Pontiller, B., Poulain, L., Quack, B., Rabe, R., Roa, J., Rolfes, S., Sanders, T., Schlangen, I., Schmidt, L., Schulz, G., Sommer, M., Thamdrup, B., Usinger, J., van Bonn, L., van Pinxteren, M., and Zhong, Q.: Biogeochemistry/atmosphere processes in the Bay of Bengal: A contribution to the 2nd International Indian Ocean Expedition, Cruise No. SO305 10 April–22 May 2024, Colombo (Sri Lanka) – Singapore (Singapore), Begutachtungspanel Forschungsschiffe, SONNE-Berichte, 86 pp., https://doi.org/10.48433/cr_so305, 2024.
Bristow, L. A., Dalsgaard, T., Tiano, L., Mills, D. B., Bertagnolli, A. D., Wright, J. J., Hallam, S. J., Ulloa, O., Canfield, D. E., Revsbech, N. P., and Thamdrup, B.: Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters, P. Natl. Acad. Sci. USA, 113, 10601–10606, https://doi.org/10.1073/pnas.1600359113, 2016.
Bristow, L. A., Callbeck, C. M., Larsen, M., Altabet, M. A., Dekaezemacker, J., Forth, M., Gauns, M., Glud, R. N., Kuypers, M. M. M., Lavik, G., Milucka, J., Naqvi, S. W. A., Pratihary, A., Revsbech, N. P., Thamdrup, B., Treusch, A. H., and Canfield, D. E.: N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone, Nat. Geosci., 10, 24–29, https://doi.org/10.1038/ngeo2847, 2017.
Brunner, B., Contreras, S., Lehmann, M. F., Matantseva, O., Rollog, M., Kalvelage, T., Klockgether, G., Lavik, G., Jetten, M. S. M., Kartal, B., and Kuypers, M. M. M.: Nitrogen isotope effects induced by anammox bacteria, P. Natl. Acad. Sci. USA, 110, 18994–18999, https://doi.org/10.1073/pnas.1310488110, 2013.
Buchwald, C. and Casciotti, K. L.: Oxygen isotopic fractionation and exchange during bacterial nitrite oxidation, Limnol. Oceanogr., 55, 1064–1074, https://doi.org/10.4319/lo.2010.55.3.1064, 2010.
Buchwald, C., Santoro, A. E., McIlvin, M. R., and Casciotti, K. L.: Oxygen isotopic composition of nitrate and nitrite produced by nitrifying cocultures and natural marine assemblages, Limnol. Oceanogr., 57, 1361–1375, https://doi.org/10.4319/lo.2012.57.5.1361, 2012.
Carvalho Junior, O. de O.: Water Masses at the Surface of the Indian Ocean, Eur. J. Environ. Earth Sci., 4, 11–21, https://doi.org/10.24018/ejgeo.2023.4.2.389, 2023.
Casciotti, K. L.: Inverse kinetic isotope fractionation during bacterial nitrite oxidation, Geochim. Cosmochim. Ac., 73, 2061–2076, https://doi.org/10.1016/j.gca.2008.12.022, 2009.
Casciotti, K. L.: Nitrogen and Oxygen Isotopic Studies of the Marine Nitrogen Cycle, Annu. Rev. Mar. Sci., 8, 379–407, https://doi.org/10.1146/annurev-marine-010213-135052, 2016.
Casciotti, K. L. and McIlvin, M. R.: Isotopic analyses of nitrate and nitrite from reference mixtures and application to Eastern Tropical North Pacific waters, Mar. Chem., 107, 184–201, https://doi.org/10.1016/j.marchem.2007.06.021, 2007.
Casciotti, K. L., Sigman, D. M., Hastings, M. G., Böhlke, J. K., and Hilkert, A.: Measurement of the Oxygen Isotopic Composition of Nitrate in Seawater and Freshwater Using the Denitrifier Method, Anal. Chem., 74, 4905–4912, https://doi.org/10.1021/ac020113w, 2002.
Casciotti, K. L., Sigman, D. M., and Ward, B. B.: Linking Diversity and Stable Isotope Fractionation in Ammonia-Oxidizing Bacteria, Geomicrobiol. J., 20, 335–353, https://doi.org/10.1080/01490450303895, 2003.
Casciotti, K. L., McIlvin, M., and Buchwald, C.: Oxygen isotopic exchange and fractionation during bacterial ammonia oxidation, Limnol. Oceanogr., 55, 753–762, https://doi.org/10.4319/lo.2010.55.2.0753, 2010.
Dalsgaard, T., Canfield, D. E., Petersen, J., Thamdrup, B., and Acuña-González, J.: N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica, Nature, 422, 606–608, https://doi.org/10.1038/nature01526, 2003.
Dalsgaard, T., Stewart, F. J., Thamdrup, B., De Brabandere, L., Revsbech, N. P., Ulloa, O., Canfield, D. E., and DeLong, E. F.: Oxygen at Nanomolar Levels Reversibly Suppresses Process Rates and Gene Expression in Anammox and Denitrification in the Oxygen Minimum Zone off Northern Chile, mBio, 5, https://doi.org/10.1128/mbio.01966-14, 2014.
Deuser, W.: Reducing environments, in: Chemical Oceanography, vol. 3, edited by: Riley, J. P. and Skirrow, G., New York, 1–37, ISBN 0-12-588606-3, 1975.
DeVries, T., Deutsch, C., Primeau, F., Chang, B., and Devol, A.: Global rates of water-column denitrification derived from nitrogen gas measurements, Nat. Geosci., 5, 547–550, https://doi.org/10.1038/ngeo1515, 2012.
Ditkovsky, S., Resplandy, L., and Busecke, J.: Unique ocean circulation pathways reshape the Indian Ocean oxygen minimum zone with warming, Biogeosciences, 20, 4711–4736, https://doi.org/10.5194/bg-20-4711-2023, 2023.
Emery, W. J.: Water Types and Water Masses, in: Encyclopedia of Ocean Sciences, 2nd edn., edited by: Steele, J. H., Academic Press, Oxford, 291–299, https://doi.org/10.1016/B978-012374473-9.00108-9, 2001.
Fawcett, S. E., Ward, B. B., Lomas, M. W., and Sigman, D. M.: Vertical decoupling of nitrate assimilation and nitrification in the Sargasso Sea, Deep-Sea Res. Pt. I, 103, 64–72, https://doi.org/10.1016/j.dsr.2015.05.004, 2015.
Felden, J., Möller, L., Schindler, U., Huber, R., Schumacher, S., Koppe, R., Diepenbroek, M., and Glöckner, F. O.: PANGAEA – Data Publisher for Earth and Environmental Science, Sci. Data, 10, 347, https://doi.org/10.1038/s41597-023-02269-x, 2023.
Gaye, B., Nagel, B., Dähnke, K., Rixen, T., and Emeis, K.-C.: Evidence of parallel denitrification and nitrite oxidation in the ODZ of the Arabian Sea from paired stable isotopes of nitrate and nitrite, Global Biogeochem. Cy., 27, 1059–1071, https://doi.org/10.1002/2011GB004115, 2013.
Gordon, A. L.: Oceanography of the Indonesian Seas and Their Throughflow, Oceanography, 18, 14–27, https://doi.org/10.5670/oceanog.2005.01, 2005.
Granger, J. and Sigman, D. M.: Removal of nitrite with sulfamic acid for nitrate N and O isotope analysis with the denitrifier method, Rapid Commun. Mass Sp., 23, 3753–3762, https://doi.org/10.1002/rcm.4307, 2009.
Granger, J. and Wankel, S.: Isotopic overprinting of nitrification on denitrification as a ubiquitous and unifying feature of environmental nitrogen cycling, P. Natl. Acad. Sci. USA, 113, E6391–E6400, https://doi.org/10.1073/pnas.1601383113, 2016.
Granger, J., Sigman, D. M., Needoba, J. A., and Harrison, P. J.: Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton, Limnol. Oceanogr., 49, 1763–1773, https://doi.org/10.4319/lo.2004.49.5.1763, 2004.
Granger, J., Sigman, D. M., Lehmann, M. F., and Tortell, P. D.: Nitrogen and oxygen isotope fractionation during dissimilatory nitrate reduction by denitrifying bacteria, Limnol. Oceanogr., 53, 2533–2545, https://doi.org/10.4319/lo.2008.53.6.2533, 2008.
Granger, J., Sigman, D. M., Rohde, M. M., Maldonado, M. T., and Tortell, P. D.: N and O isotope effects during nitrate assimilation by unicellular prokaryotic and eukaryotic plankton cultures, Geochim. Cosmochim. Ac., 74, 1030–1040, https://doi.org/10.1016/j.gca.2009.10.044, 2010.
Gruber, N.: Chapter 1 – The Marine Nitrogen Cycle: Overview and Challenges, in: Nitrogen in the Marine Environment, 2nd edn., edited by: Capone, D. G., Bronk, D. A., Mulholland, M. R., and Carpenter, E. J., Academic Press, San Diego, 1–50, https://doi.org/10.1016/B978-0-12-372522-6.00001-3, 2008.
Hansen, H. P.: Determination of oxygen, in: Methods of Seawater Analysis, edited by: Grasshoff, K. K. and Ehrhardt, M., John Wiley & Sons, Ltd, 75–89, https://doi.org/10.1002/9783527613984.ch4, 1999.
Hansen, H. P. and Koroleff, F.: Determination of nutrients, in: Methods of Seawater Analysis, edited by: Grasshoff, K. K. and Ehrhardt, M., John Wiley & Sons, Ltd, 159–228, https://doi.org/10.1002/9783527613984.ch10, 1999.
Harms, N. C., Lahajnar, N., Gaye, B., Rixen, T., Dähnke, K., Ankele, M., Schwarz-Schampera, U., and Emeis, K.-C.: Nutrient distribution and nitrogen and oxygen isotopic composition of nitrate in water masses of the subtropical southern Indian Ocean, Biogeosciences, 16, 2715–2732, https://doi.org/10.5194/bg-16-2715-2019, 2019.
Holmes, R. M., Aminot, A., Kérouel, R., Hooker, B. A., and Peterson, B. J.: A simple and precise method for measuring ammonium in marine and freshwater ecosystems, Can. J. Fish. Aquat. Sci., 56, 1801–1808, https://doi.org/10.1139/f99-128, 1999.
Howell, E. A., Doney, S. C., Fine, R. A., and Olson, D. B.: Geochemical estimates of denitrification in the Arabian Sea and the Bay of Bengal during WOCE, Geophys. Res. Lett., 24, 2549–2552, https://doi.org/10.1029/97GL01538, 1997.
Jain, V., Shankar, D., Vinayachandran, P. N., Kankonkar, A., Chatterjee, A., Amol, P., Almeida, A. M., Michael, G. S., Mukherjee, A., Chatterjee, M., Fernandes, R., Luis, R., Kamble, A., Hegde, A. K., Chatterjee, S., Das, U., and Neema, C. P.: Evidence for the existence of Persian Gulf Water and Red Sea Water in the Bay of Bengal, Clim. Dynam., 48, 3207–3226, https://doi.org/10.1007/s00382-016-3259-4, 2017.
Johnson, K. S., Riser, S. C., and Ravichandran, M.: Oxygen Variability Controls Denitrification in the Bay of Bengal Oxygen Minimum Zone, Geophys. Res. Lett., 46, 804–811, https://doi.org/10.1029/2018GL079881, 2019.
Kalvelage, T., Jensen, M. M., Contreras, S., Revsbech, N. P., Lam, P., Günter, M., LaRoche, J., Lavik, G., and Kuypers, M. M. M.: Oxygen sensitivity of anammox and coupled N-cycle processes in oxygen minimum zones, PloS One, 6, e29299, https://doi.org/10.1371/journal.pone.0029299, 2011.
Karsh, K. L., Granger, J., Kritee, K., and Sigman, D. M.: Eukaryotic Assimilatory Nitrate Reductase Fractionates N and O Isotopes with a Ratio near Unity, Environ. Sci. Technol., 46, 5727–5735, https://doi.org/10.1021/es204593q, 2012.
Kassambara, A.: ggpubr: “ggplot2” Based Publication Ready Plots, CRAN [code], https://doi.org/10.32614/CRAN.package.ggpubr, 2023.
Kawano, T.: Method for Salinity (Conductivity Ratio) Measurement, GO-SHIP Repeat Hydrogr. Man. Collect. Expert Rep. Guidel. Eds Al, IOCCP Report, https://search.oceanbestpractices.org/search?q=kawano&fields=all&activeField=all, 2010.
Kendall, C., Elliott, E. M., and Wankel, S. D.: Tracing Anthropogenic Inputs of Nitrogen to Ecosystems, in: Stable Isotopes in Ecology and Environmental Science, edited by: Michener, R. and Lajtha, K., John Wiley & Sons, Ltd, 375–449, https://doi.org/10.1002/9780470691854.ch12, 2007.
Kim, Y., Rho, T., and Kang, D.-J.: Oxygen isotope composition of seawater and salinity in the western Indian Ocean: Implications for water mass mixing, Mar. Chem., 237, 104035, https://doi.org/10.1016/j.marchem.2021.104035, 2021.
Knapp, A. N., DiFiore, P. J., Deutsch, C., Sigman, D. M., and Lipschultz, F.: Nitrate isotopic composition between Bermuda and Puerto Rico: Implications for N2 fixation in the Atlantic Ocean, Global Biogeochem. Cy., 22, GB3014, https://doi.org/10.1029/2007GB003107, 2008.
Knowles, R.: Denitrification, Microbiol. Rev., 46, 43–70, https://doi.org/10.1128/mr.46.1.43-70.1982, 1982.
Kobayashi, K., Makabe, A., Yano, M., Oshiki, M., Kindaichi, T., Casciotti, K. L., and Okabe, S.: Dual nitrogen and oxygen isotope fractionation during anaerobic ammonium oxidation by anammox bacteria, ISME J., 13, 2426–2436, https://doi.org/10.1038/s41396-019-0440-x, 2019.
Kool, D. M., Wrage, N., Oenema, O., Dolfing, J., and Van Groenigen, J. W.: Oxygen exchange between (de)nitrification intermediates and H2O and its implications for source determination of and N2O: a review, Rapid Commun. Mass Sp., 21, 3569–3578, https://doi.org/10.1002/rcm.3249, 2007.
Kumar, S., Ramesh, R., Sardesai, S., and Sheshshayee, M. S.: High new production in the Bay of Bengal: Possible causes and implications, Geophys. Res. Lett., 31, L18304, https://doi.org/10.1029/2004GL021005, 2004.
LeGrande, A. N. and Schmidt, G. A.: Global gridded data set of the oxygen isotopic composition in seawater, Geophys. Res. Lett., 33, https://doi.org/10.1029/2006GL026011, 2006.
Löscher, C. R., Mohr, W., Bange, H. W., and Canfield, D. E.: No nitrogen fixation in the Bay of Bengal?, Biogeosciences, 17, 851–864, https://doi.org/10.5194/bg-17-851-2020, 2020.
Marshall, T. A., Sigman, D. M., Beal, L. M., Foreman, A., Martínez-García, A., Blain, S., Campbell, E., Fripiat, F., Granger, R., Harris, E., Haug, G. H., Marconi, D., Oleynik, S., Rafter, P. A., Roman, R., Sinyanya, K., Smart, S. M., and Fawcett, S. E.: The Agulhas Current Transports Signals of Local and Remote Indian Ocean Nitrogen Cycling, J. Geophys. Res.-Oceans, 128, e2022JC019413, https://doi.org/10.1029/2022JC019413, 2023.
Martin, T. S. and Casciotti, K. L.: Paired N and O isotopic analysis of nitrate and nitrite in the Arabian Sea oxygen deficient zone, Deep-Sea Res. Pt. I, 121, 121–131, https://doi.org/10.1016/j.dsr.2017.01.002, 2017.
Massicotte, P. and South, A.: rnaturalearth: World Map Data from Natural Earth, CRAN [code], https://doi.org/10.32614/CRAN.package.rnaturalearth, 2025.
Naqvi, S. W. A., DeSousa, S. N., and Reddy, C. V. G.: Relationship between nutrients and dissolved oxygen with special reference to water masses in westrn Bay of Bengal, Indian J. Geo-Mar. Sci., 7, 15–17, 1978.
Naqvi, S. W. A., Yoshinari, T., Brandes, J. A., Devol, A. H., Jayakumar, D. A., Narvekar, P. V., Altabet, M. A., and Codispoti, L. A.: Nitrogen isotopic studies in the suboxic Arabian Sea, P. Indian AS.-Earth, 107, 367–378, https://doi.org/10.1007/BF02841603, 1998.
Okabe, S., Ye, S., Lan, X., Nukada, K., Zhang, H., Kobayashi, K., and Oshiki, M.: Oxygen tolerance and detoxification mechanisms of highly enriched planktonic anaerobic ammonium-oxidizing (anammox) bacteria, ISME Commun., 3, 45, https://doi.org/10.1038/s43705-023-00251-7, 2023.
Pebesma, E. and Bivand, R.: Spatial Data Science: With Applications in R, Chapman and Hall/CRC, New York, 314 pp., https://doi.org/10.1201/9780429459016, 2023.
Peng, X., Fawcett, S. E., van Oostende, N., Wolf, M. J., Marconi, D., Sigman, D. M., and Ward, B. B.: Nitrogen uptake and nitrification in the subarctic North Atlantic Ocean, Limnol. Oceanogr., 63, 1462–1487, https://doi.org/10.1002/lno.10784, 2018.
Phillips, H. E., Menezes, V. V., Nagura, M., McPhaden, M. J., Vinayachandran, P. N., and Beal, L. M.: Indian Ocean circulation?, in: The Indian Ocean and its Role in the Global Climate System, edited by: Ummenhofer, C. C. and Hood, R. R., Elsevier, 169–203, https://doi.org/10.1016/B978-0-12-822698-8.00012-3, 2024.
R Core Team: R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project (last access: 9 September 2025), 2022.
Rahman, A., Khan, M. A., Singh, A., and Kumar, S.: Hydrological characteristics of the Bay of Bengal water column using δ18O during the Indian summer monsoon, Cont. Shelf Res., 226, 104491, https://doi.org/10.1016/j.csr.2021.104491, 2021.
Rao, C. K., Naqvi, S. W. A., Kumar, M. D., Varaprasad, S. J. D., Jayakumar, D. A., George, M. D., and Singbal, S. Y. S.: Hydrochemistry of the Bay of Bengal: possible reasons for a different water-column cycling of carbon and nitrogen from the Arabian Sea, Mar. Chem., 47, 279–290, https://doi.org/10.1016/0304-4203(94)90026-4, 1994.
Rixen, T., Cowie, G., Gaye, B., Goes, J., do Rosário Gomes, H., Hood, R. R., Lachkar, Z., Schmidt, H., Segschneider, J., and Singh, A.: Reviews and syntheses: Present, past, and future of the oxygen minimum zone in the northern Indian Ocean, Biogeosciences, 17, 6051–6080, https://doi.org/10.5194/bg-17-6051-2020, 2020.
Sardessai, S., Ramaiah, N., Kumar, S., and Sousa, S. de: Influence of environmental forcings on the seasonality of dissolved oxygen and nutrients in the Bay of Bengal, J. Mar. Res., 65, 301–316, 2007.
Sarma, V. V. S. S.: An evaluation of physical and biogeochemical processes regulating the oxygen minimum zone in the water column of the Bay of Bengal, Global Biogeochem. Cy., 16, 46-1–46-10, https://doi.org/10.1029/2002GB001920, 2002.
Sarma, V. V. S. S.: Biogeochemistry of carbon, nitrogen and oxygen in the Bay of Bengal: New insights through re-analysis of data, J. Earth Syst. Sci., 131, 159, https://doi.org/10.1007/s12040-022-01915-z, 2022.
Sarma, V. V. S. S. and Udaya Bhaskar, T. V. S.: Ventilation of Oxygen to Oxygen Minimum Zone Due to Anticyclonic Eddies in the Bay of Bengal, J. Geophys. Res.-Biogeo., 123, 2145–2153, https://doi.org/10.1029/2018JG004447, 2018.
Sarma, V. V. S. S., Krishna, M. S., Viswanadham, R., Rao, G. D., Rao, V. D., Sridevi, B., Kumar, B. S. K., Prasad, V. R., Subbaiah, C. V., Acharyya, T., and Bandopadhyay, D.: Intensified oxygen minimum zone on the western shelf of Bay of Bengal during summer monsoon: influence of river discharge, J. Oceanogr., 69, 45–55, https://doi.org/10.1007/s10872-012-0156-2, 2013.
Sarma, V. V. S. S., Rao, G. D., Viswanadham, R., Sherin, C. K., Salisbury, J., Omand, M. M., Mahadevan, A., Murty, V. S. N., Shroyer, E. L., Baumgartner, M., and Stafford, K. M.: Effects of Freshwater Stratification on Nutrients, Dissolved Oxygen, and Phytoplankton in the Bay of Bengal, Oceanography, 29, 222–231, 2016.
Saxena, H., Sahoo, D., Khan, M. A., Kumar, S., Sudheer, A. K., and Singh, A.: Dinitrogen fixation rates in the Bay of Bengal during summer monsoon, Environ. Res. Commun., 2, 051007, https://doi.org/10.1088/2515-7620/ab89fa, 2020.
Schott, F. A. and McCreary, J. P.: The monsoon circulation of the Indian Ocean, Prog. Oceanogr., 51, 1–123, https://doi.org/10.1016/S0079-6611(01)00083-0, 2001.
Schott, F. A., Xie, S.-P., and McCreary Jr., J. P.: Indian Ocean circulation and climate variability, Rev. Geophys., 47, RG1002, https://doi.org/10.1029/2007RG000245, 2009.
Schulz, G. and Penopp, J.: Dual Nitrate Stable Isotopes of SONNE 305 cruise in the East Equatorial Indian Ocean and Bay of Bengal, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.983257, 2025.
Sen Gupta, R., De Sousa, S. N., and Joseph, T.: On nitrogen and phosphorus in the western Bay of Bengal, Indian J. Geo-Mar. Sci., 6, 107–110, 1977.
Sengupta, S., Parekh, A., Chakraborty, S., Ravi Kumar, K., and Bose, T.: Vertical variation of oxygen isotope in Bay of Bengal and its relationships with water masses, J. Geophys. Res.-Oceans, 118, 6411–6424, https://doi.org/10.1002/2013JC008973, 2013.
Shee, A., Sil, S., and Gangopadhyay, A.: Recent changes in the upper oceanic water masses over the Indian Ocean using Argo data, Sci. Rep.-UK, 13, 20252, https://doi.org/10.1038/s41598-023-47658-9, 2023.
Sheehan, P. M. F., Webber, B. G. M., Sanchez-Franks, A., Matthews, A. J., Heywood, K. J., and Vinayachandran, P. N.: Injection of Oxygenated Persian Gulf Water Into the Southern Bay of Bengal, Geophys. Res. Lett., 47, e2020GL087773, https://doi.org/10.1029/2020GL087773, 2020.
Shetye, S. R.: The movement and implications of the Ganges–Bramhaputra runoff on entering the Bay of Bengal, Curr. Sci. India, 64, 32–38, 1993.
Shiozaki, T., Ijichi, M., Kodama, T., Takeda, S., and Furuya, K.: Heterotrophic bacteria as major nitrogen fixers in the euphotic zone of the Indian Ocean, Global Biogeochem. Cy., 28, 1096–1110, https://doi.org/10.1002/2014GB004886, 2014.
Sigman, D. M. and Fripiat, F.: Nitrogen Isotopes in the Ocean, in: Encyclopedia of Ocean Sciences, 3rd edn., edited by: Cochran, J. K., Bokuniewicz, H. J., and Yager, P. L., Academic Press, vol. 1, 263–278, https://doi.org/10.1016/B978-0-12-409548-9.11605-7, 2019.
Sigman, D. M., Altabet, M. A., McCorkle, D. C., Francois, R., and Fischer, G.: The δ15N of nitrate in the Southern Ocean: Nitrogen cycling and circulation in the ocean interior, J. Geophys. Res.-Oceans, 105, 19599–19614, https://doi.org/10.1029/2000JC000265, 2000.
Sigman, D. M., Casciotti, K. L., Andreani, M., Barford, C., Galanter, M., and Böhlke, J. K.: A Bacterial Method for the Nitrogen Isotopic Analysis of Nitrate in Seawater and Freshwater, Anal. Chem., 73, 4145–4153, https://doi.org/10.1021/ac010088e, 2001.
Sigman, D. M., Granger, J., DiFiore, P. J., Lehmann, M. M., Ho, R., Cane, G., and van Geen, A.: Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin, Global Biogeochem. Cy., 19, https://doi.org/10.1029/2005GB002458, 2005.
Sigman, D. M., DiFiore, P. J., Hain, M. P., Deutsch, C., Wang, Y., Karl, D. M., Knapp, A. N., Lehmann, M. F., and Pantoja, S.: The dual isotopes of deep nitrate as a constraint on the cycle and budget of oceanic fixed nitrogen, Deep-Sea Res. Pt. I, 56, 1419–1439, https://doi.org/10.1016/j.dsr.2009.04.007, 2009.
Snider, D. M., Spoelstra, J., Schiff, S. L., and Venkiteswaran, J. J.: Stable Oxygen Isotope Ratios of Nitrate Produced from Nitrification: 18O-Labeled Water Incubations of Agricultural and Temperate Forest Soils, Environ. Sci. Technol., 44, 5358–5364, https://doi.org/10.1021/es1002567, 2010.
South, A., Michael, S., and Massicotte, P.: rnaturalearthdata: World Vector Map Data from Natural Earth Used in “rnaturalearth”, CRAN [code], https://doi.org/10.32614/CRAN.package.rnaturalearthdata, 2025.
Sprintall, J. and Tomczak, M.: On the formation of central water and thermocline ventilation in the southern hemisphere, Deep-Sea Res. Pt. I, 40, 827–848, https://doi.org/10.1016/0967-0637(93)90074-D, 1993.
Sprintall, J., Wijffels, S. E., Molcard, R., and Jaya, I.: Direct estimates of the Indonesian Throughflow entering the Indian Ocean: 2004–2006, J. Geophys. Res.-Oceans, 114, C07001, https://doi.org/10.1029/2008JC005257, 2009.
Sridevi, B. and Sarma, V. V. S. S.: A revisit to the regulation of oxygen minimum zone in the Bay of Bengal, J. Earth Syst. Sci., 129, 107, https://doi.org/10.1007/s12040-020-1376-2, 2020.
Srivastava, R., Ramesh, R., Prakash, S., Anilkumar, N., and Sudhakar, M.: Oxygen isotope and salinity variations in the Indian sector of the Southern Ocean, Geophys. Res. Lett., 34, https://doi.org/10.1029/2007GL031790, 2007.
Strous, M., Fuerst, J. A., Kramer, E. H. M., Logemann, S., Muyzer, G., van de Pas-Schoonen, K. T., Webb, R., Kuenen, J. G., and Jetten, M. S. M.: Missing lithotroph identified as new planctomycete, Nature, 400, 446–449, https://doi.org/10.1038/22749, 1999.
Sun, X., Frey, C., Garcia-Robledo, E., Jayakumar, A., and Ward, B. B.: Microbial niche differentiation explains nitrite oxidation in marine oxygen minimum zones, ISME J., 15, 1317–1329, https://doi.org/10.1038/s41396-020-00852-3, 2021.
Sun, X., Frey, C., and Ward, B. B.: Nitrite Oxidation Across the Full Oxygen Spectrum in the Ocean, Global Biogeochem. Cy., 37, e2022GB007548, https://doi.org/10.1029/2022GB007548, 2023.
Sverdrup, H. U., Johnson, M. W., and Fleming, R. H.: The Oceans Their Physics, Chemistry, and General Biology, Prentice-Hall, New York, 1060 pp., 1942.
Tiedje, J. M.: Ecology of denitrification and dissimilatory nitrate reduction to ammonium, in: Biology of anaerobic microorganisms, edited by: Zehnder, A. J. B., John Wiley and Sons, New York, 179–244, 1988.
Tomczak, M. and Godfrey, J. S.: Chapter 11 – The Indian Ocean, in: Regional Oceanography, edited by: Tomczak, M. and Godfrey, J. S., Pergamon, Amsterdam, 193–220, https://doi.org/10.1016/B978-0-08-041021-0.50015-1, 1994.
Trimmer, M., Nicholls, J. C., and Deflandre, B.: Anaerobic Ammonium Oxidation Measured in Sediments along the Thames Estuary, United Kingdom, Appl. Environ. Microb., 69, 6447–6454, https://doi.org/10.1128/AEM.69.11.6447-6454.2003, 2003.
Ummenhofer, C. C. and Hood, R. R. (Eds.): The Indian Ocean and its Role in the Global Climate System, 1st edn., Elsevier, ISBN 978-0-12-822698-8, 2024.
Vinayachandran, P. N., Masumoto, Y., Mikawa, T., and Yamagata, T.: Intrusion of the Southwest Monsoon Current into the Bay of Bengal, J. Geophys. Res.-Oceans, 104, 11077–11085, https://doi.org/10.1029/1999JC900035, 1999.
Wankel, S. D., Kendall, C., Pennington, J. T., Chavez, F. P., and Paytan, A.: Nitrification in the euphotic zone as evidenced by nitrate dual isotopic composition: Observations from Monterey Bay, California, Global Biogeochem. Cy., 21, https://doi.org/10.1029/2006GB002723, 2007.
Ward, B. B.: How Nitrogen Is Lost, Science, 341, 352–353, https://doi.org/10.1126/science.1240314, 2013.
Wickham, H., Chang, W., Henry, L., Pedersen, T. L., Takahashi, K., Wilke, C., Woo, K., Yutani, H., Dunnington, D., and van den Brand, T.: ggplot2: Elegant Graphics for Data Analysis, CRAN [code], https://doi.org/10.32614/CRAN.package.ggplot2, 2016.
Winkler, L. W.: Die Bestimmung des im Wasser gelösten Sauerstoffes, Ber. Dtsch. Chem. Ges., 21, 2843–2854, https://doi.org/10.1002/cber.188802102122, 1888.
Wyrtki, K.: Oceanographic atlas of the International Indian Ocean Expedition, National Science Foundation, Washington, DC, 531 pp., 1971.
Wyrtki, K.: An equatorial jet in the Indian ocean, Science, 181, 262–264, https://doi.org/10.1126/science.181.4096.262, 1973.
You, Y.: Seasonal variations of thermocline circulation and ventilation in the Indian Ocean, J. Geophys. Res.-Oceans, 102, 10391–10422, https://doi.org/10.1029/96JC03600, 1997.
You, Y. and Tomczak, M.: Thermocline circulation and ventilation in the Indian Ocean derived from water mass analysis, Deep-Sea Res. Pt. I, 40, 13–56, https://doi.org/10.1016/0967-0637(93)90052-5, 1993.




