the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Warming enhances carbon dioxide and methane fluxes from Red Sea seagrass (Halophila stipulacea) sediments
Celina Burkholz
Neus Garcias-Bonet
Carlos M. Duarte
Seagrass meadows are autotrophic ecosystems acting as carbon sinks, but they have also been shown to be sources of carbon dioxide (CO2) and methane (CH4). Seagrasses can be negatively affected by increasing seawater temperatures, but the effects of warming on CO2 and CH4 fluxes in seagrass meadows have not yet been reported. Here, we examine the effect of two disturbances on air–seawater fluxes of CO2 and CH4 in Red Sea Halophila stipulacea communities compared to adjacent unvegetated sediments using cavity ring-down spectroscopy. We first characterized CO2 and CH4 fluxes in vegetated and adjacent unvegetated sediments, and then experimentally examined their response, along with that of the carbon (C) isotopic signature of CO2 and CH4, to gradual warming from 25 ∘C (winter seawater temperature) to 37 ∘C, 2 ∘C above current maximum temperature. In addition, we assessed the response to prolonged darkness, thereby providing insights into the possible role of suppressing plant photosynthesis in supporting CO2 and CH4 fluxes. We detected 6-fold-higher CO2 fluxes in vegetated compared to bare sediments, as well as 10- to 100-fold-higher CH4 fluxes. Warming led to an increase in net CO2 and CH4 fluxes, reaching average fluxes of 10 422.18 ± 2570.12 µmol CO2 m−2 d−1 and 88.11±15.19 µmol CH4 m−2 d−1, while CO2 and CH4 fluxes decreased over time in sediments maintained at 25 ∘C. Prolonged darkness led to an increase in CO2 fluxes but a decrease in CH4 fluxes in vegetated sediments. These results add to previous research identifying Red Sea seagrass meadows as a significant source of CH4, while also indicating that sublethal warming may lead to increased emissions of greenhouse gases from seagrass meadows, providing a feedback mechanism that may contribute to further enhancing global warming.
- Article
(2078 KB) - Full-text XML
-
Supplement
(142 KB) - BibTeX
- EndNote
Global warming, as a result of anthropogenic emissions of greenhouse gases, has led to ocean warming by 0.11 ∘C between 1971 to 2010 (IPCC, 2014), with the global mean sea-surface temperature predicted to increase further with additional emissions, depending on emission scenarios (IPCC, 2014). Ocean warming is leading to a shift in species and ecosystem processes (Hoegh-Guldberg and Bruno, 2010), including metabolic processes that are under strong thermal control (Brown et al., 2004; Garcias-Bonet et al., 2018, 2019b).
Ecosystem metabolism can also be a source of greenhouse gases, depending on the metabolic balance of the community, where autotrophic communities (net community production (NCP) >0) act as a sink for carbon dioxide (CO2), while heterotrophic communities (NCP <0) act as a source of CO2 (Duarte et al., 2011). Since respiration rates tend to increase faster with warming than primary production does (Brown et al., 2004; Harris et al., 2006; Regaudie-De-Gioux and Duarte, 2012), warming may lead to the shifting of typically autotrophic ecosystems, such as seagrass meadows, to being net heterotrophic, thereby switching from acting as sinks to sources of CO2 (Harris et al., 2006). Emissions of metabolic greenhouse gases with ocean warming may provide a feedback mechanism by which anthropogenic emissions of greenhouse gases may lead to warming-dependent emissions by coastal ecosystems, therefore enhancing climate warming. This feedback effect is particularly likely to occur where methane (CH4) is released, as CH4 is calculated to have a global warming potential 28 times larger than CO2 per mole of carbon (C) emitted (Myhre et al., 2013).
Indeed, CO2 and CH4 emissions from some tropical mangrove forests have been calculated and found to partially offset the capacity of mangroves to act as C sinks (Rosentreter et al., 2018). Whereas the emission of CO2 and CH4 from seagrass ecosystems has received far less attention, seagrass ecosystems have been reported to support CH4 emissions of the order of 1.4 to 401.3 µmol CH4 m−2 d−1 (cf. Table 1 in Garcias-Bonet and Duarte, 2017). Provided estimates of their global extent of seagrass meadows ranging from a documented 326 000 km2 (Unsworth et al., 2019) to a predicted 1.6×106 km2 (Jayathilake and Costello, 2018), seagrass meadows may be important yet hitherto overlooked contributors to CH4 emissions. Garcias-Bonet and Duarte (2017) reported that seagrasses could contribute to global CH4 emissions by releasing CH4 at a rate of 0.09 to 2.7 Tg yr−1, which may increase the contribution of marine global emissions to previously reported global estimates by about 30 % (Garcias-Bonet and Duarte, 2017).
Seagrasses are known to be autotrophic ecosystems, acting as C sinks (Duarte et al., 2010) supporting a global burial rate of 27.4 Tg C yr−1 (Duarte et al., 2005b). They store carbon in their below- and aboveground biomass in the short term, as well as in their sediment in the long term (Duarte et al., 2005b). They account for 10 % of the C storage in ocean sediments even though they only cover 0.2 % of the ocean surface (Duarte et al., 2005b; Fourqurean et al., 2012). However, disturbances can lead to the loss of biomass and the emissions of stored C turning blue carbon ecosystems into C sources (Macreadie et al., 2015; Lovelock et al., 2017; Arias-Ortiz et al., 2018), which will ultimately contribute to global emissions intensifying the greenhouse effect. Lyimo et al. (2018) showed that stressors such as shading and grazing led to an increase of CH4 emissions by seagrass ecosystems by reducing their photosynthetic capacity. Garcias-Bonet and Duarte (2017) reported that CH4 release from seagrass sediments tended to increase with seawater temperature and suggested that CH4 emissions by seagrass ecosystems may be under temperature control in the Red Sea. Indeed, some seagrass ecosystems in the Red Sea have been shown to shift from an autotrophic to a heterotrophic state during the warmer summer months, indicating that some seagrass communities might already grow past their thermal optimum (Burkholz et al., 2019a).
The Red Sea ranks as the warmest sea in the world, with summer seawater temperatures reaching 35 ∘C, and is warming at higher rates (0.17±0.07 ∘C decade−1; Chaidez et al., 2017) than those of the global ocean (0.11 ∘C decade−1; Rhein et al., 2013). Provided respiration rates and also CH4 fluxes in seagrass ecosystems are likely to increase with temperature, seagrass meadows in the Red Sea may be close to shifting from net sinks to net sources of greenhouse gases with further warming. Emission rates are also dependent on organic carbon supply, as high organic matter in sediments can promote an increase in CH4 production (Sotomayor et al., 1994; Gonsalves et al., 2011) and organic matter released from seagrass photosynthesis may also stimulate CO2 and CH4 production in the sediment community. Indeed, sediments in seagrass ecosystems support a 1.7-fold increase in organic matter content compared to surrounding bare sediments, not only due to the slow turnover of biomass but also due to their ability to trap particles (Duarte et al., 2005a; Kennedy et al., 2010).
Here, we test the hypothesis that CO2 and CH4 fluxes by seagrass communities increase with warming. We do so by experimentally examining the effect of warming and plant activity on air–seawater fluxes of CO2 and CH4 in a Red Sea seagrass (Halophila stipulacea) community. The tropical seagrass species Halophila stipulacea (Forsskål) Ascherson is native to the Indian Ocean and is one of the most common species in the Red Sea (Qurban et al., 2019). It seems to be highly adaptive to various environments, as it is now found as an exotic species in the Mediterranean (Lipkin, 1975) and the Caribbean Sea (Ruiz and Ballantine, 2004), indicating its high resilience to changing conditions (Por, 1971). We first characterize air–seawater fluxes of CO2 and CH4 in Red Sea Halophila stipulacea communities compared to adjacent unvegetated sediments and then experimentally examine their response, along with that of the C isotopic signature of CO2 and CH4, to gradual warming from 25 to 37 ∘C. In addition, we assess the response to prolonged darkness, thereby providing insights into the possible role of plant photosynthesis in supporting CO2 and CH4 fluxes.
2.1 Study site and sample collection
Samples were collected at Al Kharar, a lagoon on the Saudi coast of the central Red Sea in February 2018. Two H. stipulacea meadows at a depth of 2–3 m, S1 (22∘56′46.5′′ N, 38∘52′40.6′′ E) and S2 (22∘54′44.5′′ N, 38∘53′50.9′′ E), were chosen to represent a range of organic matter content in the sediment and were selected to evaluate greenhouse gas fluxes. Moreover, the H. stipulacea meadow in the middle of the lagoon (S2) with higher biomass density (Table 1) was chosen as the study site to experimentally assess the role of temperature and darkness in greenhouse gas fluxes. The seagrass and sediment community was sampled using translucent cylindrical polyvinyl chloride (PVC) cores (26 cm length and 9.5 cm in diameter). The sharpened edge of the core was carefully pushed approximately 10 cm into the sediment with a rubber hammer so that the structure of leaves, roots and sediment stayed intact. A rubber stopper was then placed on top, before the core was carefully pulled out of the sediment without disturbing the structure and another rubber stopper was placed on the bottom of the core. The sediment cores were immediately transported to the laboratory.
2.2 Sediment and plant characterization
Once the cores were opened, the first 10 cm of the sediment and the plant biomass from the same cores were collected and dried at 60 ∘C to a constant dry weight (DW). To characterize the two different H. stipulacea meadows, sediment and plant biomass samples were then ground to conduct sediment composition and nutrient analyses. A 50 mL tube was filled with sediment from the first 10 cm, and the contents were dried at 60 ∘C to a constant dry weight and weighed to determine the sediment bulk density (g sediment cm−3). Organic matter content was analyzed by loss on ignition (LOI; Dean, 1974). Approximately 5 g of dried sediment was placed in a muffle furnace and burned at 450 ∘C for 5 h. The organic matter content was calculated as
The carbonate content was estimated using a Pressure Gauge Calcimeter. Approximately 1 g of sample was placed in the calcimeter, and the recipient was filled with 10 % hydrochloric acid (HCl). The mass of CaCO3 in the sample (g) was then calculated as follows:
where p is the pressure recorded (ppm), b is the slope and a the intercept derived from the calibration curve, and w is the exact weight of each sediment sample (g). The percentage of CaCO3 in the sample (% DW) was then calculated using Eq. (3):
Dried sediment and plant samples were digested using US Environmental Protection Agency (EPA) method 3052 and analyzed with nitric acid (HNO3) and HCl using US EPA method 200.7 following manufacturer instructions. The phosphorus content (% DW) was analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES) on an Agilent 5110 ICP-OES (Agilent Technologies, Santa Clara, CA, USA). The C and nitrogen (N) concentration of both plants and sediments was analyzed after acidification with HCl (Hedges and Stern, 1984), using a FLASH 2000 Organic Elemental Analyzer (CHNS/O-2, Thermo Fisher Scientific, Waltham, MA, USA). The isotopic signature of 13C in sediment organic matter was analyzed, using cavity ring-down spectroscopy (CRDS G2201-I, Picarro Inc., Santa Clara, CA, USA), from the 13C of CO2 released by a combustion module (Costech Analytical Technologies Inc., CA, USA) delivering the CO2 resulting from combusting the sediment organic matter to the CRDS instrument.
2.3 Assessment of carbon dioxide and methane air–seawater fluxes
In February 2018, triplicate cores from vegetated and adjacent bare (about 5 m from the edge of the seagrass patch) sediments were collected from sites S1 and S2 and transferred to incubation chambers (Percival Scientific Inc., Perry, IA, USA) set at 25 ∘C with a 12 h light (up to 70 µmol photons m−2 s−1) : 12 h dark (12 h L : 12 h D) cycle to measure the greenhouse gas (CO2 and CH4) fluxes supported by these communities. Before measuring fluxes, the water overlying the sediment inside the cores was carefully siphoned until only 5 mm of water remained over the sediment surface; fresh seawater was carefully siphoned in the core to avoid disturbing the redoxcline, leaving a headspace of approximately 5–6 cm; and the cores were closed again with stoppers containing gas-tight valves. The cores were left for 1 h to allow for equilibration between the seawater and the headspace air. We then sampled 10 mL of air from each core using a syringe and injected the air sample in a cavity ring-down spectrometer (Picarro Inc., Santa Clara, CA, USA) through a Small Sample Isotopic Module extension (SSIM A0314, Picarro Inc., Santa Clara, CA, USA), which provided both the partial pressure and the isotopic carbon composition of the CO2 and CH4 in the air sample. One sample from each core was taken at the start (T0), after 12 h of light (T1) and after 12 h of dark (T2). The daily CO2 and CH4 fluxes were calculated from the difference between T0 and T2 taking into account the core surface area (µmol m−2 d−1). Before and after each sampling, two standards were measured (A: 750 ppm CO2, 9.7 ppm CH4; B: 250.5 ppm CO2, 3.25 ppm CH4).
2.4 Effect of warming on carbon dioxide and methane air–seawater fluxes
In March 2018, we collected 18 vegetated and 18 bare sediment cores from site S2 to evaluate the response of greenhouse gas fluxes to warming. The sampling was performed as described above. The cores were transferred to the Coastal and Marine Resources Core Lab (CMOR, KAUST, Saudi Arabia). Nine vegetated and nine bare sediment cores each were placed in two aquaria with flow-through seawater set at in situ temperature (25 ∘C) and with a 12 h L (up to 200 µmol photons m−2 s−1) : 12 h D cycle. One aquarium was maintained at 25 ∘C over the entire duration of the experiment to serve as a control for temperature-independent variability in fluxes. The temperature in the second aquarium was increased at a rate of 1 ∘C d−1 to allow for acclimatization of the vegetated and bare cores. CO2 and CH4 fluxes were measured at every 2 ∘C from 25 to 37 ∘C, with parallel measurements conducted on the cores maintained at 25 ∘C. After a 1 d acclimation period at each new temperature, the cores were closed with the stoppers and transferred to incubation chambers (Percival Scientific Inc., Perry, IA, USA) set at the target temperature for CO2 and CH4 flux measurements as described above. After the 24 h measurements, the cores were returned to the aquaria. An additional core kept at each of the constant temperature and warming sets was sampled every 4 d (i.e., at 4 ∘C temperature intervals in the warming treatment) to analyze sediment composition. The cores used for flux estimates were opened after the final measurement (20 d since collection) to estimate the plant biomass and analyze the sediment composition at the end of the experiment.
2.5 Effect of darkness on carbon dioxide and methane air–seawater fluxes
In May 2018, six vegetated and six bare sediment cores were collected from site S2 and kept at a constant 25 ∘C with a 24 h dark cycle. Only during the measurements in the incubators were the cores exposed to a 12 h L : 12 h D cycle, allowing fluxes to be compared with those measured in cores permanently maintained with the 12 h L : 12 h D photoperiod. CO2 and CH4 fluxes were measured after the first day of acclimation and then kept in the aquaria until signs of seagrass mortality started to become apparent, which occurred after 1 week in the dark. CO2 and CH4 fluxes were measured at alternate days as detailed above. At the end of the experiment (21 d since collection), the cores were opened and sampled to assess plant biomass and sediment composition.
2.6 Measurements of carbon dioxide and methane air–seawater fluxes
The concentration of CO2 in the seawater after equilibrium was calculated based on the concentration of CO2 in the headspace (ppm) measured by CRDS according to Sea et al. (2018) and Wilson et al. (2012):
where β is the Bunsen solubility coefficient of CO2 (mol mL−1 atm−1), ma is the CO2 concentration measured in the headspace (ppm) and pdry is the atmospheric pressure of dry air (atm). The Bunsen solubility coefficient of CO2 was calculated using Eq. (5):
where Hcp is the Henry constant (mol mL−1 atm−1) calculated using the marelac R package (Soetaert et al., 2016). R is the ideal gas constant (0.082057459 atm L mol mL−1 K−1), and T is the standard temperature (273.15 K).
The atmospheric pressure of dry air (pdry) was calculated as follows:
where pwet is the atmospheric pressure of wet air. Boyle's law was applied as gas was collected several times from the same core.
The concentration of dissolved CO2 in seawater before equilibrium was then calculated using Eq. (7):
where Vw is the volume of seawater (mL) and Va is the volume of the headspace (mL). The units were then converted to nanomolar (nM):
The daily CO2 fluxes were calculated from the difference between T0 and T2 taking into account the core surface area (µmol m−2 d−1).
Daily CH4 fluxes were estimated using the same calculations as for the CO2 fluxes with the exception of the Bunsen solubility coefficient. The Bunsen solubility coefficient was calculated as a function of the seawater temperature and salinity following Wiesenburg and Guinasso (1979). The total CO2 greenhouse-equivalent fluxes were calculated assuming CH4 to have a greenhouse potential 28-fold greater than that of CO2 per mol of C (Myhre et al., 2013).
2.7 Isotopic composition of carbon dioxide (δ13C-CO2) and methane (δ13C-CH4)
The isotopic signature of CO2 and CH4 produced during the incubations was estimated using Keeling plots following Garcias-Bonet and Duarte (2017). δ13C of CO2 and CH4 produced in our incubations was extracted from the intercept of the linear regression between the inverse of the gas concentration (ppm−1) and the isotopic signature measured from the discrete samples in the CDRS instrument.
2.8 Data analysis
The data were analyzed for normality using the Shapiro–Wilk test. The Mann–Whitney test and Student's t test were used to test for differences in seagrass and sediment composition between sites and between vegetated and bare sediments, and analysis of variation (ANOVA) and the Kruskal–Wallis test were used to test for differences between vegetated and bare sediments and both sites. To assess differences in greenhouse gas fluxes between different H. stipulacea communities, differences in CO2 and CH4 fluxes were analyzed between sites and between vegetated and bare sediment by using the Kruskal–Wallis test. Trends in the flux between the communities experiencing warming and the ones maintained at 25 ∘C, as well as in the isotopic signature of δ13C-CO2 and δ13C-CH4 over time, were analyzed by linear regression. When assessing the effect of darkness on greenhouse gas fluxes, the trend of CO2 and CH4 fluxes and their isotopic signatures were analyzed by linear regression. The statistical analyses were conducted in PRISM 5 (GraphPad Software, La Jolla, CA, USA) and JMP Pro 13.1.0 (SAS Institute Inc., Cary, NC, USA). The data are openly available from Burkholz et al. (2019b).
3.1 Seagrass and sediment composition
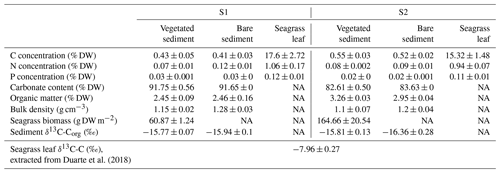
Carbon, nitrogen and phosphorus (P) concentrations in seagrass leaves were low, but they were 4- to 40-fold higher than vegetated and bare sediment concentrations (Table 1). Seagrass sampled in site S1 had the highest C, N and P concentrations in the leaves, while sediment C and P concentrations differed significantly between sites (ANOVA, p<0.05, and Kruskal–Wallis, p<0.001, respectively), with the highest C and the lowest P concentrations found in the sediment of S2 (Table 1). There were no consistent differences in C, N and P concentration in bare and vegetated sediments (Table 1).
The sediments had high, but variable, carbonate concentrations, which differed between sites (Kruskal–Wallis, p<0.0001; Table 1). The organic matter content was slightly increased in S2 compared to S1, in both vegetated (t test, p<0.0001) and bare (t test, p<0.001) sediments (Table 1). Sediment bulk density was similar in both S1 and S2 sites, but vegetated sediments in S1 showed significantly lower bulk density compared to bare sediments (t test, p<0.05; Table 1). Seagrass biomass density was significantly higher in S2 compared to S1 (t test, p<0.05). The isotopic signature of sediment organic carbon ranged across sites from ‰, in vegetated sediments, to ‰, in bare sediments (Table 1). The carbon isotopic signature of seagrass leaves from the same location has been recently reported as ‰ by Duarte et al. (2018).
3.2 Carbon dioxide and methane air–seawater fluxes
The daily CO2 flux was up to 6-fold higher in vegetated compared to bare sediments and tended to be generally higher in S2 compared to S1, where bare sediments showed net CO2 uptake, although differences were not significant (Kruskal–Wallis, p>0.05; Fig. 1a). At both sites, S1 and S2, the daily net CH4 flux was 10- to 100-fold higher in vegetated compared to adjacent bare sediments with generally higher fluxes at S2 (Kruskal–Wallis, p<0.01; Fig. 1b). The total CO2 equivalent (CO2-eq) fluxes varied between sites and were higher in the vegetated compared to the bare sediments (Kruskal–Wallis, p<0.01; Fig. 1c).
3.3 Effect of warming on carbon dioxide and methane air–seawater fluxes
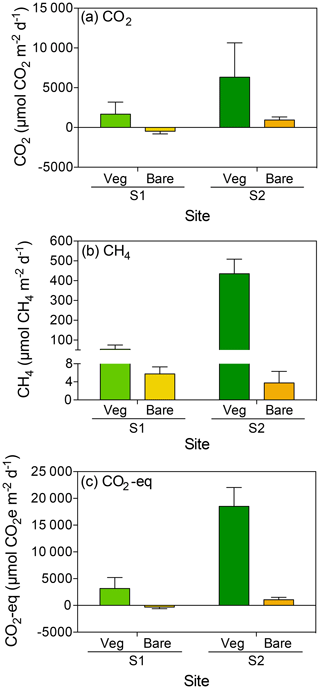
The CO2 fluxes in vegetated sediments increased greatly with warming (R2=0.38, p<0.001) but decreased over time when the community was maintained at 25 ∘C (R2=0.30, p<0.01; Fig. 2a, Table S1 in the Supplement), shifting from sediments showing net CO2 emission to net CO2 uptake. Similar responses were observed in the bare sediments, where CO2 fluxes increased with warming (R2=0.54, p<0.0001), while the community tended to shift over time from supporting net CO2 emission to net CO2 uptake when maintained at 25 ∘C (Fig. 2b). The net CO2 flux – i.e., the difference between the CO2 fluxes in warming sediments and those at sediments maintained at 25 ∘C – increased significantly with warming in both the vegetated and the bare sediment (R2=0.74 and p<0.05, and R2=0.91 and p<0.001, respectively; Fig. 2c).
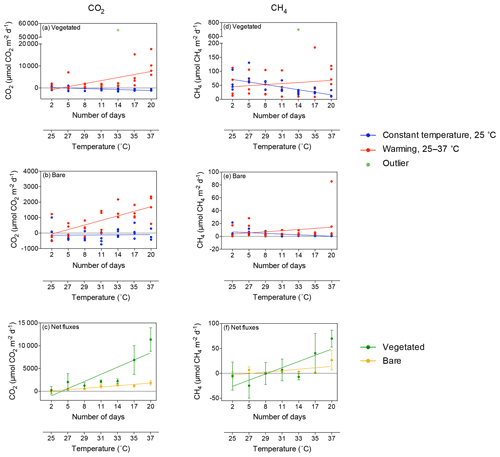
Figure 2Mean ± SE CO2 (left) and CH4 (right) fluxes in (a, d) vegetated and (b, e) bare sediments. Symbols indicate each replicate of the community experiencing warming from 25 to 37 ∘C (red) and the community maintained at 25 ∘C (blue) over the experimental period (number of days since the onset of the experiment). An outlier at 33 ∘C in vegetated sediments in shown in green. (c, f) Mean ± SE (c) CO2 and (f) CH4 net fluxes in vegetated (green) and bare (yellow) sediments over the experimental period (number of days since the onset of the experiment). The second x axis indicates the experimental temperature for the community exposed to warming from 25 to 37 ∘C. Lines represent a fitted linear equation.
CH4 fluxes declined over time when the sediments were maintained at 25 ∘C, both in vegetated (R2=0.43, p<0.001; Fig. 2d) and, less strongly, bare sediments (R2=0.24, p<0.01; Fig. 2e, Table S2). In contrast, CH4 fluxes tended to increase with temperature in vegetated (Fig. 2d) and bare (Fig. 2e) sediments gradually warmed from 25 to 37 ∘C, although it was not significant (p>0.05 and p>0.05, respectively; Table S2). The net CH4 fluxes – i.e., the difference between the CH4 fluxes in sediments exposed to warming and sediments maintained at 25 ∘C – increased significantly over time (i.e., with warming) in vegetated (R2=0.69, p<0.05) but not in bare sediments (p>0.05; Fig. 2f). An outlier in the vegetated sediment at 33 ∘C supporting extreme emissions (CO2 flux of 55 170 µmol CO2 m−2 d−1 and CH4 flux of 699.8 CH4 µmol m−2 d−1) was observed on day 14 in one of the replicates of the warming treatment where seagrass had died (Fig. 2a and d); it was excluded from the regression analyses reported above.
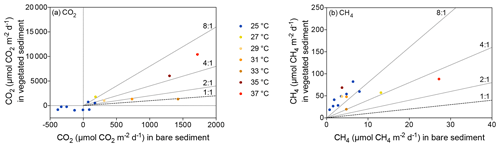
Despite CO2 and CH4 fluxes showing the same response to warming in both types of sediment, vegetated sediments held higher fluxes than bare sediments. The relationship between net CO2 and CH4 fluxes in bare vs. vegetated sediments showed that both bare and vegetated communities tended to act as net CO2 sinks at 25 ∘C, but tended to act as CO2 sources at warmer temperatures (Fig. 3a), whereas net CH4 fluxes were 3- to 8-fold higher in vegetated compared to bare sediments (Fig. 3b). The CH4 / CO2 ratio declined in the vegetated sediments exposed to warming from 7 to 0.8 %. For CO2 and CH4 fluxes in vegetated sediments, the Q10 value for the temperature range 25–37 ∘C was 9 and 1.5, respectively, while the Q10 value for bare sediments was 13.8 and 4.2, respectively.
3.4 Effect of darkness on carbon dioxide and methane air–seawater fluxes
The vegetated sediment shifted over time from showing net CO2 uptake to net CO2 emission when maintained in the dark (R2=0.70, p<0.05), while the increase in the bare sediment was not significant (p>0.05; Fig. 4a, Table S3). In contrast, when vegetated sediment was maintained at 25 ∘C with a 12 h L : 12 h D photoperiod, the community shifted from net CO2 emission to net CO2 uptake (Mann–Whitney, p<0.05; Fig. 5a). In bare sediments, CO2 fluxes showed the same trend in cores maintained at 25 ∘C with a 12 h L : 12 h D photoperiod as under dark conditions (Fig. 5b).
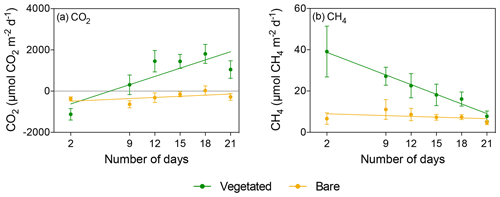
Figure 4Mean ± SE (a) CO2 and (b) CH4 fluxes in vegetated (green) and bare (yellow) sediments of communities exposed to prolonged darkness over the experimental period (number of days since the onset of the experiment).
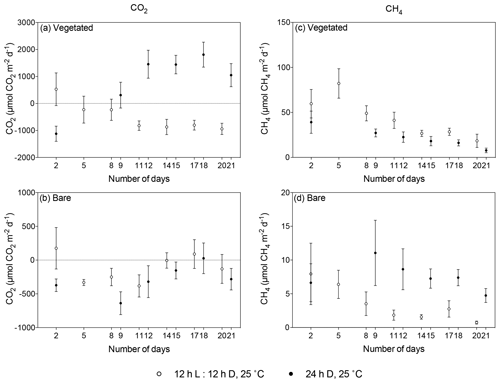
Figure 5Comparison of mean ± SE CO2 (left) and CH4 (right) fluxes in (a, c) vegetated and (b, d) bare sediments maintained at 25 ∘C with a 12 h L : 12 h D photoperiod (white) and communities kept at 25 ∘C with a 24 h D period (black) over the experimental period (number of days since the onset of the experiment). Dots indicate mean values, and error bars indicate standard error of the mean.
When vegetated sediments were kept in the dark, net CH4 fluxes decreased 5-fold over time (R2=0.99, p<0.0001; Fig. 4b, Table S4). However, the CH4 fluxes did not differ significantly between vegetated cores maintained at 25 ∘C in the 12 h L : 12 h D photoperiod or in the dark (Mann–Whitney, p>0.05), showing the same trend of decreasing CH4 fluxes (Fig. 5c). In the bare sediment, CH4 fluxes in sediments kept in the dark were higher than those at 25 ∘C with a 12 h L : 12 h D photoperiod, with significant differences only observed on days 14 and 20 (Mann–Whitney, p<0.05 and p<0.05, respectively; Fig. 5d).
3.5 Isotopic composition of carbon dioxide (δ13C-CO2) and methane (δ13C-CH4)
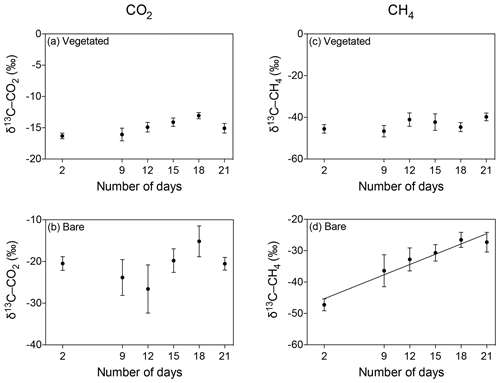
The isotopic signature of the δ13C-CO2 became heavier with warming in the bare sediment, increasing from ‰ δ13C at 25 ∘C to ‰ δ13C at 37 ∘C (R2=0.91, p<0.001), while the other treatments showed similar values over time, ranging from a minimum average of ‰ to a maximum average of ‰ δ13C (Fig. 6a–d).
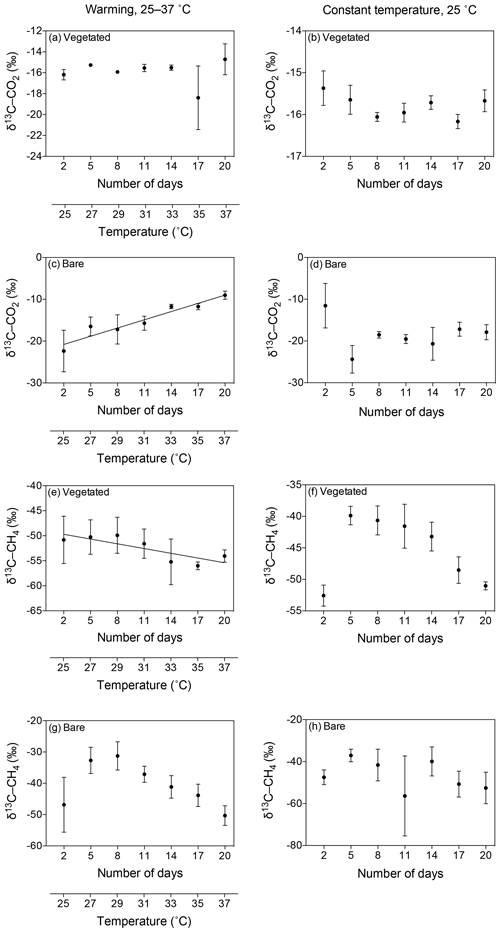
Figure 6Mean ± SE isotopic signature of CO2 (δ13C-CO2) and CH4 (δ13C-CH4) in the communities experiencing warming from 25 to 37 ∘C (left) and the communities maintained at 25 ∘C (right). (a–d) δ13C-CO2 is shown for the (a, b) vegetated and (c, d) bare sediments over the experimental period (number of days since the onset of the experiment). (e–h) δ13C-CH4 is shown for the (e, f) vegetated and (g, h) bare sediment over the experimental period. The second x axis indicates the temperature increase for the community experiencing warming.
The isotopic signature of δ13C-CH4 decreased over time in both vegetated and bare sediments, whether they were maintained at constant temperature or experienced warming (Fig. 6e–h). The isotopic signature in the vegetated sediment exposed to warming decreased significantly from −50.8 ‰ to −54.06 ‰ (R2=0.67, p<0.001).
The δ13C isotopic composition of both CO2 and CH4 became heavier over time when the community was kept in the dark (Fig. 7), with a significant increase of δ13C-CH4 in bare sediments (R2=0.94, p<0.01; Fig. 7d).
4.1 Carbon dioxide and methane air–seawater fluxes
The values reported for CO2 and CH4 fluxes varied greatly between the two sites studied here, with higher fluxes in the more organic sediments with higher biomass density (S2). CO2 and CH4 fluxes were also highly variable over time at the studied site, as the first evaluation of fluxes at the same location delivered rates up to 100-fold above the rates of the second measurement 1 week later. Hence, organic matter availability along with temperature may account for the large variation in CO2 and CH4 fluxes. Additionally, the variability of CO2 and CH4 fluxes could also be supported by infaunal species present in the cores that were not recorded in this study. These trends were similar to results reported in previous studies, as a high variability between species and locations was found (cf. Table 1 in Garcias-Bonet and Duarte, 2017).
Even though there were some differences, carbon, nitrogen and phosphorus concentrations were generally similar, and they did not seem to have an effect on CO2 and CH4 fluxes. Carbon, nitrogen and phosphorus concentrations were low compared to mean values (carbon: 33.6±0.31 % DW; nitrogen: 1.92±0.05 % DW; phosphorus: 0.23±0.011 % DW) reported for seagrass leaves by Duarte (1990). Serrano et al. (2018) explained the discrepancy between Red Sea data and global data with the extreme conditions in the Red Sea, such as low nutrient input and high temperatures, as well as a limited data set favoring high carbon stocks in the Mediterranean.
The results presented here add to the findings of Garcias-Bonet and Duarte (2017) identifying Red Sea seagrass communities as a significant source of CH4. The presence of seagrass resulted in a larger organic matter supply to the sediments, favoring the presence of methanogens, which led to higher CH4 fluxes compared to those fluxes supported in bare sediments (Barber and Carlson, 1993; Bahlmann et al., 2015), consistent with the up-to-100-fold-higher CH4 fluxes supported by vegetated compared to bare sediments in this study. Additionally, higher fluxes in vegetated cores could be an indicator of direct effects resulting from the presence of seagrass, as vascular plants on land have been shown to have varying effects on methane emissions caused by differences in biomass and gross photosynthesis (Öquist and Svensson, 2002).
Similar trends were also seen by Garcias-Bonet and Duarte (2017), who reported an increase in CH4 fluxes with increasing organic matter content in Red Sea seagrass sediments. They reported organic matter contents in Red Sea seagrass sediments ranging from 2.33±0.07 % (Halodule uninervis) to 12.42±0.23 % (Enhalus acoroides), including a mixed meadow with H. stipulacea and H. uninervis showing slightly increased organic matter content of 3.51±0.17 % compared to vegetated sediments at S2. Moreover, they found the highest CH4 fluxes in meadows with the highest biomass, confirming our findings of higher fluxes at study site S2.
In terms of CO2 equivalents, only the bare sediment maintained at 25 ∘C seemed to act as a C sink over the experimental period, while the vegetated sediments, both maintained at 25 ∘C and exposed to warming, acted as sources of greenhouse gases. A sublethal disturbance, such as warming below the lethal threshold, can therefore lead to a shift of seagrass ecosystems from acting as net sinks to net sources of greenhouse gases, as demonstrated experimentally here.
4.2 Effect of warming
Both CO2 and CH4 fluxes were higher in vegetated compared to adjacent bare sediments, indicating elevated remineralization rates in vegetated sediments as well as a higher susceptibility of seagrass sediment to increasing temperatures. Vegetated sediments exposed to warming shifted from acting as a CO2 sink to an increasingly intense source, while the CO2 fluxes in vegetated sediments maintained at 25 ∘C decreased over time. Warming leads to an increase in both community photosynthesis and respiration, with respiration's faster rate of increase (Harris et al., 2006) explaining the shift to a CO2 source in sediments exposed to a thermal stressor. However, the fluxes maintained at 25 ∘C showed a net CO2 uptake with a mean of 464.78±156.6 µmol CO2 m−2 d−1 (Table S1), while those reported in a mixed Halodule sp. and Halophila sp. meadow in India showed a net CO2 release (dry season: 1190±1600 µmol CO2 m−2 d−1; wet season: 18 400 ± 8800 µmol CO2 m−2 d−1; Banerjee et al., 2018). Both values reported were measured at higher temperatures (dry season: 30±0.68 ∘C; wet season: 27.94±0.72 ∘C; Banerjee et al., 2018) compared to our fluxes measured at 25 ∘C, also indicating that temperature might lead to higher fluxes. An initial high CO2 flux measured on day two after sampling could be an indicator for the experienced disturbance due to sample collection and transportation even though we allowed the cores some time to adapt.
Mean CH4 fluxes at in situ temperature (25 ∘C) in vegetated sediments were lower than the mean value of 85.1±27.8 µmol CH4 m−2 d−1 reported for other seagrass meadows in the Red Sea (Garcias-Bonet and Duarte, 2017). In contrast, the community exposed to warming reached a maximum average CH4 flux almost 4-fold higher than the community held at 25 ∘C and showed a clear increase with warming relative to sediments held at 25 ∘C. The increase in CH4 fluxes with warming was consistent with reports from Barber and Carlson (1993) for a Thalassia testudinum community in Florida Bay and from Garcias-Bonet and Duarte (2017) for Red Sea seagrass communities, who reported higher CH4 fluxes at higher temperatures. Additionally, previous research has shown that methanogenesis has a higher thermal dependence than respiration and photosynthesis (Yvon-Durocher et al., 2014), confirming the trends seen here with increasing fluxes at higher temperatures. We also reported a 10-fold decline in CH4 fluxes over time for sediment communities maintained at 25 ∘C, which could be attributable to increased sulfate reduction, reduced CH4 production or a combination of both. Methane is produced under anoxic conditions in marine sediments, yet only a small portion is released, as CH4 production by methanogens is compensated for by CH4 oxidation by sulfate-reducing bacteria (Barnes and Goldberg, 1976). Similar to the trends seen in CO2 fluxes, the decrease in CH4 fluxes could be attributable to an initial stress response to the disturbance caused by sample collection and transportation. While reduced photosynthetic activity and a degradation in biomass could result in higher CH4 fluxes (Lyimo et al., 2018), the cores maintained at 25 ∘C might show the effect of healthy conditions.
Increasing water temperature led to a decrease in the CH4 / CO2 ratio. While there was ∼7 % of sequestered carbon released as CH4 to the atmosphere in vegetated sediments at 25 ∘C (on day two ), it decreased to ∼0.8 % in vegetated sediments at 37 ∘C. In contrast, Banerjee et al. (2018) reported ∼1 % of carbon being released as CH4.
The isotopic composition of CO2 in all treatments showed generally heavier isotopic signatures compared to previous reports of seagrass carbon (average δ13C value of ‰ for Red Sea seagrass and ‰ for H. stipulacea in the Red Sea; Duarte et al., 2018), indicating various organic matter sources such as macroalgae blades (13.38±0.3 ‰), mangrove leaves (26.58±0.13 ‰) and seston (25.43±0.42 ‰; Duarte et al., 2018). However, the mean δ13C value of Red Sea seagrass sediments was reported to be ‰ (Garcias-Bonet et al., 2019a), similar to the results found in this study. Our chosen study sites were located in an enclosed lagoon with a high abundance of mangrove forests, leading to the conclusion that mangroves might be a major source of organic matter for our study sites. However, a recent study applying stable isotope mixing models found the major contributors to the organic matter in seagrass sediments in the Red Sea to be seagrass leaves and macroalgae blades, with contributions of 43 % and 37 %, respectively (Garcias-Bonet et al., 2019a).
The isotopic signature of CO2 released from bare sediments shifted with warming, suggesting a shift from seston, mangroves and macroalgae to seagrass carbon as the source of CO2. In the vegetated cores, the isotopic composition of CO2 stayed rather constant, indicating several sources of organic carbon with no clear shift, regardless of warming.
The isotopic signature of CH4 in vegetated sediments confirmed its biogenic source as previous reports have shown that the isotopic signature of CH4 from biogenic sources can range from −40 ‰ to −80 ‰, while the isotopic signature of CH4 from geological and thermogenic sources ranges from −30 ‰ to −50 ‰ (Reeburgh, 2014). The isotopic composition of CH4 in bare sediments was generally at the lower end of this range, with no clear shift with increasing temperature.
The isotopic composition of CH4 can be determined by the production of CH4 (methanogenesis) leading to lower δ13C values and the oxidation of CH4 (methanotrophy) leading to higher δ13C values (Whiticar, 1990). Garcias-Bonet and Duarte (2017) reported fluctuations in the isotopic signature of CH4 in Red Sea seagrass meadows, suggesting an indication of both processes. With exposure to increasing temperatures, we observed a shift to a lighter isotopic signature of CH4 in vegetated sediments, thereby indicating an increasing CH4 production by methanogens with warming.
4.3 Effect of prolonged darkness
Communities maintained at 25 ∘C with a 12 h L : 12 h D photoperiod showed continuous net CO2 uptake, while the communities kept in the dark shifted, as expected, to a heterotrophic state, acting as a CO2 source. The net CO2 production corresponded to community respiration rates, while that with a 12 h L : 12 h D photoperiod corresponded to the net community production.
We found, however, no effect of prolonged darkness on CH4 fluxes, suggesting that the elevated CH4 fluxes in vegetated sediments were not directly supported by fresh photosynthetic products, but rather by the elevated organic matter content in vegetated sediments compared to bare ones. These findings were in contrast to those reported by Lyimo et al. (2018), who found increased CH4 fluxes under shading, indicating that degradation of belowground biomass might have been the key factor related to increased CH4 fluxes. However, they also reported varying results for different shading intensities, with low intensity having similar fluxes compared to their control group (Lyimo et al., 2018). In contrast, Öquist and Svensson (2002) found that photosynthesis might be regulating methane fluxes in a subarctic peatland ecosystem, with lower photosynthesis resulting in lower methane fluxes.
4.4 Implications
Reports on greenhouse gas fluxes by seagrass ecosystems are limited (Oremland, 1975; Barber and Carlson, 1993; Alongi et al., 2008; Deborde et al., 2010; Bahlmann et al., 2015; Garcias-Bonet and Duarte, 2017; Banerjee et al., 2018; Lyimo et al., 2018), and no reports had been previously published on how increasing seawater temperatures might affect greenhouse gas fluxes by seagrass ecosystems. Blue carbon ecosystems have been shown to turn into C sources when disturbances lead to mortality (Macreadie et al., 2015; Lovelock et al., 2017; Arias-Ortiz et al., 2018), consistent with the very large CO2 and CH4 fluxes observed in one vegetated sediment where the seagrass died when warmed to 33 ∘C. However, even where seagrass remained alive, warming led to elevated greenhouse fluxes. Additionally, the elevated nutrient and high organic matter stock in seagrass meadows, which supports 1.7 times more organic matter content than surrounding bare sediments, can promote an increase in CO2 and CH4 fluxes following disturbance (Gonsalves et al., 2011; Sotomayor et al., 1994). Our results suggest that this stock in seagrass sediments may be remineralized to support net greenhouse gas fluxes at the warmer temperatures reached and with further warming of the Red Sea. Hence, warming may, as other disturbances (Lovelock et al., 2017), shift seagrass ecosystems from net sinks to net sources of greenhouse gases, thereby providing a feedback mechanism that may contribute to further enhancing global warming.
In summary, this study reports, for the first time, experimental evidence that warming leads to increased greenhouse gas (CO2 and CH4) fluxes in a H. stipulacea meadow in the Red Sea, and it may lead to seagrass meadows shifting from acting as sinks to sources of greenhouse gases. Increased fluxes at higher temperatures can be an indication of higher re-mineralization rates and a higher susceptibility of vegetated sediments to temperature. The elevated organic matter content, higher biomass density and greater plant activity in vegetated sediments led to increased CO2 and CH4 fluxes in vegetated compared to bare sediments and a much steeper increase in CO2 and CH4 fluxes with warming. In addition, prolonged darkness led to an increase in CO2 fluxes, while CH4 fluxes decreased over time, also indicating organic matter to be the driver. However, we also found a high variability in fluxes over time, indicating that other factors, such as infaunal species, could play a role as well. While the current focus is on conserving blue carbon ecosystems from losses due to deteriorated water quality or mechanical damage, our results show that sublethal warming may also lead to emissions of greenhouse gases from seagrass meadows, contributing to a feedback between ocean warming and further climate change.
All data are accessible in the repository Pangea (https://doi.org/10.1594/PANGAEA.905687, Burkholz et al., 2019b).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-1717-2020-supplement.
NGB, CMD and CB designed the project. CB collected the samples and conducted the experiments. NGB, CMD and CB analyzed the results; CMD and CB wrote the first draft of the manuscript; and all authors contributed substantially to the final draft. All authors approved the final submission.
The authors declare that they have no conflict of interest.
We thank Paloma Carrillo de Albornoz, Mongi Ennasri and Vijayalaxmi Dasari for their help with analyses. We also thank Katherine Rowe and the Coastal and Marine Resources Core Lab (CMOR) for their assistance during field work and CMOR for their support with the experimental setup.
This research has been supported by the King Abdullah University of Science and Technology through baseline and CARF funding (grant nos. FCC/1/1973-32-01 and BAS/1/1071-01-01).
This paper was edited by Aninda Mazumdar and reviewed by two anonymous referees.
Alongi, D. M., Trott, L. A., Undu, M. C., and Tirendi, F.: Benthic microbial metabolism in seagrass meadows along a carbonate gradient in Sulawesi, Indonesia, Aquat. Microb. Ecol., 51, 141–152, https://doi.org/10.3354/ame01191, 2008.
Arias-Ortiz, A., Serrano, O., Masqué, P., Lavery, P. S., Mueller, U., Kendrick, G. A., Rozaimi, M., Esteban, A., Fourqurean, J. W., Marbà, N., Mateo, M. A., Murray, K., Rule, M. J., and Duarte, C. M.: A marine heatwave drives massive losses from the world's largest seagrass carbon stocks, Nat. Clim. Change, 8, 338–344, https://doi.org/10.1038/s41558-018-0096-y, 2018.
Bahlmann, E., Weinberg, I., Lavrič, J. V., Eckhardt, T., Michaelis, W., Santos, R., and Seifert, R.: Tidal controls on trace gas dynamics in a seagrass meadow of the Ria Formosa lagoon (southern Portugal), Biogeosciences, 12, 1683–1696, https://doi.org/10.5194/bg-12-1683-2015, 2015.
Banerjee, K., Paneerselvam, A., Ramachandran, P., Ganguly, D., Singh, G., and Ramesh, R.: Seagrass and macrophyte mediated CO2 and CH4 dynamics in shallow coastal waters, PLoS One, 13, e0203922, https://doi.org/10.1371/journal.pone.0203922, 2018.
Barber, T. R. and Carlson, P. R. J.: Effects of seagrass die-off on benthic fluxes and porewater concentrations of CO2, H2S, and CH4 in Florida Bay sediments, in: Biogeochemistry of Global Change, edited by: Oremland, R. S., 530–550, Springer, Boston, MA, USA, 1993.
Barnes, R. O. and Goldberg, E. D.: Methane production and consumption in anoxic marine sediments, Geology, 4, 297–300, https://doi.org/10.1130/0091-7613(1976)4<297:MPACIA>2.0.CO;2, 1976.
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B.: Toward a metabolic theory of ecology, Ecology, 85, 1771–1789, https://doi.org/10.1890/03-9000, 2004.
Burkholz, C., Duarte, C. M., and Garcias-Bonet, N.: Thermal dependence of seagrass ecosystem metabolism in the Red Sea, Mar. Ecol.-Prog. Ser., 614, 79–90, https://doi.org/10.3354/meps12912, 2019a.
Burkholz, C., Garcias-Bonet, N., and Duarte, C. M.: Carbon dioxide and methane fluxes from Red Sea seagrass sediments, PANGAEA, https://doi.org/10.1594/PANGAEA.905687, 2019b.
Chaidez, V., Dreano, D., Agusti, S., Duarte, C. M., and Hoteit, I.: Decadal trends in Red Sea maximum surface temperature, Sci. Rep.-UK, 7, 8144, https://doi.org/10.1038/s41598-017-08146-z, 2017.
Dean, W. E. J.: Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods, J. Sediment. Petrol., 44, 242–248, https://doi.org/10.1306/74D729D2-2B21-11D7-8648000102C1865D, 1974.
Deborde, J., Anschutz, P., Guérin, F., Poirier, D., Marty, D., Boucher, G., Thouzeau, G., Canton, M., and Abril, G.: Methane sources, sinks and fluxes in a temperate tidal Lagoon: The Arcachon lagoon (SW France), Estuar. Coast. Shelf S., 89, 256–266, https://doi.org/10.1016/j.ecss.2010.07.013, 2010.
Duarte, C. M.: Seagrass nutrient content, Mar. Ecol.-Prog. Ser., 67, 201–207, https://doi.org/10.3354/meps067201, 1990.
Duarte, C. M., Holmer, M., and Marbà, N.: Plant-microbe interactions in seagrass meadows, in: Interactions between macro- and microorganisms in marine sediments, edited by: Kristensen, E., Haese, R. R., and Kostka, J. E., 31–60, American Geophysical Union, Washington, D.C., 2005a.
Duarte, C. M., Middelburg, J. J., and Caraco, N.: Major role of marine vegetation on the oceanic carbon cycle, Biogeosciences, 2, 1–8, https://doi.org/10.5194/bg-2-1-2005, 2005b.
Duarte, C. M., Marbà, N., Gacia, E., Fourqurean, J. W., Beggins, J., Barrón, C., and Apostolaki, E. T.: Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows, Global Biogeochem. Cy., 24, 1–9, https://doi.org/10.1029/2010GB003793, 2010.
Duarte, C. M., Agusti, S., and Regaudie-de-Gioux, A.: The role of marine biota in the biogeochemical and geological cycles of carbon, in: The role of marine biota in the functioning of the biosphere, edited by: Duarte, C. M., 39–54, Fundación BBVA, Madrid, 2011.
Duarte, C. M., Delgado-Huertas, A., Anton, A., Carrillo-de-Albornoz, P., López-Sandoval, D. C., Agustí, S., Almahasheer, H., Marbá, N., Hendriks, I. E., Krause-Jensen, D., and Garcias-Bonet, N.: Stable isotope (δ13C, δ15N, δ18O, δD) composition and nutrient concentration of Red Sea primary producers, Front. Mar. Sci., 5, 1–12, https://doi.org/10.3389/fmars.2018.00298, 2018.
Fourqurean, J. W., Duarte, C. M., Kennedy, H., Marbà, N., Holmer, M., Mateo, M. A., Apostolaki, E. T., Kendrick, G. A., Krause-Jensen, D., McGlathery, K. J., and Serrano, O.: Seagrass ecosystems as a globally significant carbon stock, Nat. Geosci., 5, 505–509, https://doi.org/10.1038/ngeo1477, 2012.
Garcias-Bonet, N. and Duarte, C. M.: Methane production by seagrass ecosystems in the Red Sea, Front. Mar. Sci., 4, 1–10, https://doi.org/10.3389/fmars.2017.00340, 2017.
Garcias-Bonet, N., Fusi, M., Ali, M., Shaw, D. R., Saikaly, P. E., Daffonchio, D., and Duarte, C. M.: High denitrification and anaerobic ammonium oxidation contributes to net nitrogen loss in a seagrass ecosystem in the central Red Sea, Biogeosciences, 15, 7333–7346, https://doi.org/10.5194/bg-15-7333-2018, 2018.
Garcias-Bonet, N., Delgado-Huertas, A., Carrillo-de-Albornoz, P., Anton, A., Almahasheer, H., Marbà, N., Hendriks, I. E., Krause-Jensen, D., and Duarte, C. M.: Carbon and nitrogen concentrations, stocks, and isotopic compositions in red sea seagrass and mangrove sediments, Front. Mar. Sci., 6, 1–12, https://doi.org/10.3389/fmars.2019.00267, 2019a.
Garcias-Bonet, N., Vaquer-Sunyer, R., Duarte, C. M., and Marbà, N.: Warming effect on nitrogen fixation in Mediterranean macrophyte sediments, Biogeosciences, 16, 167–175, https://doi.org/10.5194/bg-16-167-2019, 2019b.
Gonsalves, M. J., Fernandes, C. E. G., Fernandes, S. O., Kirchman, D. L., and Loka Bharathi, P. A.: Effects of composition of labile organic matter on biogenic production of methane in the coastal sediments of the Arabian Sea, Environ. Monit. Assess., 182, 385–395, https://doi.org/10.1007/s10661-011-1883-3, 2011.
Harris, L. A., Duarte, C. M., and Nixon, S. W.: Allometric laws and prediction in estuarine and coastal ecology, Estuar. Coast., 29, 340–344, https://doi.org/10.1007/BF02782002, 2006.
Hedges, J. I. and Stern, J. H.: Carbon and nitrogen determinations of carbonate-containing solids, Limnol. Oceanogr., 29, 657–663, https://doi.org/10.4319/lo.1984.29.3.0657, 1984.
Hoegh-Guldberg, O. and Bruno, J. F.: The impact of climate change on the world's marine ecosystems, Science, 328, 1523–1529, https://doi.org/10.1126/science.1189930, 2010.
IPCC: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Pachauri, R. K. and Meyer, L. A., p. 151, IPCC, Geneva, Switzerland, 2014.
Jayathilake, D. R. and Costello, M. J.: A modelled global distribution of the seagrass biome, Biol. Conserv., 226, 120–126, https://doi.org/10.1016/j.biocon.2018.07.009, 2018.
Kennedy, H., Beggins, J., Duarte, C. M., Fourqurean, J. W., Holmer, M., Marbá, N., and Middelburg, J. J.: Seagrass sediments as a global carbon sink: Isotopic constraints, Global Biogeochem. Cy., 24, 1–8, https://doi.org/10.1029/2010GB003848, 2010.
Lipkin, Y.: Halophila stipulacea, a review of a successful immigration, Aquat. Bot., 1, 203–215, https://doi.org/10.1016/0304-3770(75)90023-6, 1975.
Lovelock, C. E., Atwood, T., Baldock, J., Duarte, C. M., Hickey, S., Lavery, P. S., Masque, P., Macreadie, P. I., Ricart, A. M., Serrano, O., and Steven, A.: Assessing the risk of carbon dioxide emissions from blue carbon ecosystems, Front. Ecol. Environ., 15, 257–265, https://doi.org/10.1002/fee.1491, 2017.
Lyimo, L. D., Gullström, M., Lyimo, T. J., Deyanova, D., Dahl, M., Hamisi, M. I., and Björk, M.: Shading and simulated grazing increase the sulphide pool and methane emission in a tropical seagrass meadow, Mar. Pollut. Bull., 134, 89–93, https://doi.org/10.1016/j.marpolbul.2017.09.005, 2018.
Macreadie, P. I., Trevathan-Tackett, S. M., Skilbeck, C. G., Sanderman, J., Curlevski, N., Jacobsen, G., and Seymour, J. R.: Losses and recovery of organic carbon from a seagrass ecosystem following disturbance, P. R. Soc. B, 282, 1–6, https://doi.org/10.1098/rspb.2015.1537, 2015.
Myhre, G., Shindell, D., Bréon, F.-M., Collins, W., Fuglestvedt, J., Huang, J., Koch, D., Lamarque, J.-F., Lee, D., Mendoza, B., Nakajima, T., Robock, A., Stephens, G., Takemura, T., and Zhang, H.: Anthropogenic and Natural Radiative Forcing, in: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V., and Midgley, P. M., 659–740, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2013.
Öquist, M. G. and Svensson, B. H.: Vascular plants as regulators of methane emissions from a subarctic mire ecosystem, J. Geophys. Res.-Atmos., 107, 1–10, https://doi.org/10.1029/2001JD001030, 2002.
Oremland, R. S.: Methane production in shallow-water, tropical marine sediments, Appl. Microbiol., 30, 602–608, 1975.
Por, F. D.: One hundred years of Suez Canal – A century of Lessepsian migration: retrospect and viewpoints, Syst. Zool., 20, 138–159, https://doi.org/10.2307/2412054, 1971.
Qurban, M. A. B., Karuppasamy, M., Krishnakumar, P. K., Garcias-Bonet, N., and Duarte, C. M.: Seagrass distribution, composition and abundance along the Saudi Arabian coast of Red Sea, in: Oceanographic and biological aspects of the Red Sea, edited by: Rasul, N. and Stewart, I., 367–385, Springer, Cham., 2019.
Reeburgh, W. S.: Global Methane Biogeochemistry, in: Treatise On Geochemistry, edited by: Holland, H. D. and Turekian, K. K., 71–94, Elsevier, Oxford, 2014.
Regaudie-De-Gioux, A. and Duarte, C. M.: Temperature dependence of planktonic metabolism in the ocean, Global Biogeochem. Cy., 26, 1–10, https://doi.org/10.1029/2010GB003907, 2012.
Rhein, M., Rintoul, S. R., Aoki, S., Campos, E., Chambers, D., Feely, R. A., Gulev, S., Johnson, G. C., Josey, S. A., Kostianoy, A., Mauritzen, C., Roemmich, D., Talley, L. D., and Wang, F.: Observations: Ocean, in: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., and Bos, J., 255–315, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2013.
Rosentreter, J. A., Maher, D. T., Erler, D. V., Murray, R. H., and Eyre, B. D.: Methane emissions partially offset “blue carbon” burial in mangroves, Sci. Adv., 4, eaao4985, https://doi.org/10.1126/sciadv.aao4985, 2018.
Ruiz, H. and Ballantine, D. L.: Occurrence of the seagrass Halophila stipulacea in the tropical West Atlantic, B. Mar. Sci., 75, 131–135, 2004.
Sea, M. A., Garcias-Bonet, N., Saderne, V., and Duarte, C. M.: Carbon dioxide and methane fluxes at the air–sea interface of Red Sea mangroves, Biogeosciences, 15, 5365–5375, https://doi.org/10.5194/bg-15-5365-2018, 2018.
Serrano, O., Almahasheer, H., Duarte, C. M., and Irigoien, X.: Carbon stocks and accumulation rates in Red Sea seagrass meadows, Sci. Rep.-UK, 8, 15037, https://doi.org/10.1038/s41598-018-33182-8, 2018.
Soetaert, K., Petzoldt, T., Meysman, F., and Meire L.: marelac: Tools for Aquatic Sciences, R package, available at: https://cran.r-project.org/web/packages/marelac/marelac.pdf (last access: 19 November 2018), 2016.
Sotomayor, D., Corredor, J. E., and Morell, J. M.: Methane flux from mangrove sediments along the Southwestern Coast of Puerto Rico, Coast. Estuar. Res. Fed., 17, 140–147, https://doi.org/10.2307/1352563, 1994.
Unsworth, R. K., McKenzie, L. J., Collier, C. J., Cullen-Unsworth, L. C., Duarte, C. M., Eklöf, J. S., Jarvis, J. C., Jones, B. L., and Nordlund, L. M.: Global challenges for seagrass conservation, Ambio, 48, 801–815, https://doi.org/10.1007/s13280-018-1115-y, 2019.
Whiticar, M. J.: A geochemial perspective of natural gas and atmospheric methane, Org. Geochem., 16, 531–547, https://doi.org/10.1016/0146-6380(90)90068-B, 1990.
Wiesenburg, D. A. and Guinasso, N. L. J.: Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water, J. Chem. Eng. Data, 24, 356–360, https://doi.org/10.1021/je60083a006, 1979.
Wilson, S. T., Böttjer, D., Church, M. J., and Karl, D. M.: Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic north pacific ocean, Appl. Environ. Microb., 78, 6516–6523, https://doi.org/10.1128/AEM.01146-12, 2012.
Yvon-Durocher, G., Allen, A. P., Bastviken, D., Conrad, R., Gudasz, C., St-Pierre, A., Thanh-Duc, N., and Del Giorgio, P. A.: Methane fluxes show consistent temperature dependence across microbial to ecosystem scales, Nature, 507, 488–491, https://doi.org/10.1038/nature13164, 2014.