the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Distribution and flux of dissolved iron in the peatland-draining rivers and estuaries of Sarawak, Malaysian Borneo
Xiaohui Zhang
Moritz Müller
Shan Jiang
Ying Wu
Xunchi Zhu
Aazani Mujahid
Zhuoyi Zhu
Mohd Fakharuddin Muhamad
Edwin Sien Aun Sia
Faddrine Holt Ajon Jang
Jing Zhang
Dissolved iron (dFe) is essential for multiple biogeochemical reactions in oceans, such as photosynthesis, respiration and nitrogen fixation. Currently, large uncertainties remain regarding the input of riverine dFe into coastal oceans, especially in tropical rivers in southeastern Asia. In the present study, the concentrations of dFe and distribution patterns of dFe were determined along the salinity gradient in the Rajang River and three blackwater rivers that drain from peatlands, including the Maludam River, the Sebuyau River and the Simunjan River. In the Rajang River, the dFe concentration in freshwater samples (salinity <1 PSU – practical salinity units) in the wet season (March 2017) was higher than that in the dry season (August 2016), which might be related to the resuspension of sediment particles and soil erosion from cropland. In the Rajang estuary, an intense removal of dFe in low-salinity waters (salinity <15 PSU) was observed, which was likely due to salt-induced flocculation and absorption of dFe onto suspended particulate matter (SPM). However, increases in the dFe concentration in the wet season were also found, which may be related to dFe desorption from SPM and the influences of agricultural activities. In the blackwater rivers, the dFe concentration reached 44.2 µmol L−1, indicating a strong contribution to the dFe budget from peatland leaching. The dFe flux derived from the Rajang estuary to the South China Sea was estimated to be kg yr−1. For blackwater rivers, the dFe flux was approximately kg yr−1 in the Maludam River. Anthropogenic activities may play an important role in the dFe yield, such as in the Serendeng tributary of the Rajang River and Simunjan River, where intensive oil palm plantations were observed.
- Article
(2709 KB) - Full-text XML
-
Supplement
(715 KB) - BibTeX
- EndNote
Iron (Fe) is an essential element for enzymes and is deemed to be responsible for photosynthesis, respiration and nitrogen fixation (Moore et al., 2009; Raven, 1988; Williams, 1981). Over the past 4 decades, Fe has been identified as a micronutrient that significantly supports primary productivity in oceans (Brand and Sunda, 1983; Moore et al., 2009; Tagliabue et al., 2017). In particular, after a series of in situ fertilization experiments, researchers have verified the occurrence of Fe limitation on the growth of phytoplankton and its critical effect on CO2 fixation (Boyd et al., 2007; Martin, 1990).
At the global scale, the amount of riverine dissolved iron (dFe) transported to coastal oceans is estimated to be 1.5×109 mol yr−1 (Boyd and Ellwood, 2010; de Baar and de Jong, 2001; Jickells et al., 2005; Milliman and Farnsworth, 2011; Saitoh et al., 2008). Tropical rivers might contribute a significant quantity of dFe based on studies of the Amazon River (Bergquist and Boyle, 2006; Gaillardet et al., 1997) and the Congo River (Coynel et al., 2005; Dupré et al., 1996). However, few studies have assessed the dFe concentrations and transport patterns of tropical rivers in southeastern Asia, even though those rivers can account for approximately 30 % of fluvial discharge to oceans (Milliman and Farnsworth, 2011).
Estuaries, which are the interaction zone between surface loading and coastal oceans, effectively modulate dFe concentrations during mixing and hence change the magnitude of the riverine dFe flux. A wide range of studies on the behaviors of dFe in estuaries have been conducted, and several distribution patterns have been documented (Boyle et al., 1977; Herzog et al., 2017; Oldham et al., 2017; Zhu et al., 2018). Generally, estuaries act as a sink for dFe due to flocculation occurring between cations and high-molecular-weight colloids (Bergquist and Boyle, 2006; Boyle et al., 1977; Stolpe and Hassellov, 2007). In some rivers with high concentrations of dissolved organic matter (DOM), dFe has been found to be conservative because of the chemical connection of Fe to DOM (Oldham et al., 2017; Sanders et al., 2015; Stolpe et al., 2010). The magnitude of dFe removal from estuaries can be quantified by removal factors (RFs). Anthropogenic activities related to coal mining, the ore industry and agriculture activities could significantly impact the concentrations and distributions of dFe in estuaries (Braungardt et al., 2003; Morillo et al., 2005; Xue et al., 2016).
Currently, only limited records of dFe concentrations have been provided for peatland-draining rivers (Batchelli et al., 2010; Krachler et al., 2010; Oldham et al., 2017). The dFe distribution in peatland-draining estuaries is also largely unknown. Coastal belts in southeastern Asia are covered by a large area of peatlands, reaching approximately 9 % of the global peatland coverage (Dommain et al., 2011; Joosten, 2012). To the best of the authors' knowledge, the dFe concentrations in Malaysia have only been determined (1) in Pelagus, where high concentrations of dFe were observed in freshwater due to the sediments there (Siong, 2015), and (2) in Bebar, a blackwater river in Pahang, Malaysia, where the concentration of dFe was up to 30 µmol L−1. However, information about the distribution and biogeochemistry of dFe is lacking (Shuhaimiothman et al., 2009). Such limitations in dFe data may markedly influence the regional estimations of the dFe budget.
To fill this gap in knowledge, two cruises were conducted in the state of Sarawak, Borneo, Malaysia, which included the largest river in the state of Sarawak (the Rajang River) and three blackwater rivers. This study aims to determine (1) the concentration and distribution of dFe in the studied rivers and their estuaries, (2) the seasonal variation in the concentration and distribution of dFe in the Rajang River and its estuary, and (3) the dFe yield and the magnitude of riverine dFe flux to the coastal areas.
2.1 Study area
Malaysia has the second largest peatland area (approximately 2.6×104 km2) in southeastern Asia (Mutalib et al., 1992). The state of Sarawak accounts for the largest peatland area of Malaysia and has widespread blackwater rivers (Joosten, 2012; Wetlands International, 2010). Approximately 23 % of the peatland in Malaysia is defined as relatively undisturbed, of which 17 % is in Sarawak (Wetlands International, 2010). Since the mid-1980s, the rubber, textile, metal, food processing, petroleum and electronic industries have been developed in the area, and they are the major economic supporters in Malaysia (Trade Chakra, 2009). As a response, the deforestation rate in Sarawak increased to 2 % per year from 1990 to 2010 (Miettinen et al., 2012), and this rate is attributed to oil palm and rubber tree plantations (Joosten, 2012).
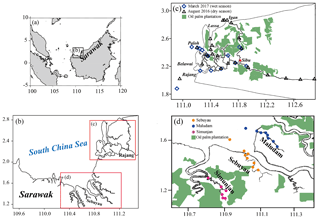
Figure 1Distribution of sample stations in Sarawak (b), Malaysia (a), including Rajang in August 2016 and Rajang, Maludam, Sebuyau and Simunjan in March 2017. In (c) and (d), the green feature layer was redrawn by the dataset from Global Forest Watch (http://gfw2-data.s3.amazonaws.com/country/mys/zip/mys_oil_palm.zip, last access: 12 January 2020).
The Rajang River, i.e., the largest river in Malaysia, which has a length of 530 km, flows from Iran Mountain to the South China Sea (Fig. 1a, b). The drainage basin is 51×103 km2 (Milliman and Farnsworth, 2011; Staub and Esterle, 1993). The drainage area of the Rajang estuary is 6500 km2, and 50 % of it is covered with extensive peat at depths greater than 3 m (Staub and Gastaldo, 2003). The Rajang River is approximately 5–10 and 8–20 m deep during the dry season and the wet season, respectively. The mainstream flow velocity ranges from 0.2 to 0.6 and 0.8 to 1.2 m s−1 during the dry season and the wet season, respectively (Tawan et al., 2019). The discharge rate for the Rajang River reaches 6000 m3 s−1 in the wet season (December to March), with an average discharge of approximately 3600 m3 s−1 (Staub et al., 2000; Staub and Gastaldo, 2003). The climate in the Rajang watershed is classified as the tropical ever-wet type (Morley and Flenley, 1987), while the precipitation rate varies between the dry and wet seasons. The city of Sibu is assumed to be the boundary line of the Rajang drainage basin and Rajang estuary according to physiographic conditions (Staub et al., 2000; Staub and Esterle, 1993), and saltwater intrusions can reach downstream of the city (Jiang et al., 2019). Apart from mineral soils transported from the upper stream, the Rajang estuary also receives materials from the adjacent hill regions and the Retus River (Staub and Gastaldo, 2003). There are several tributaries for the Rajang River in the estuary, including Igan, Hulu Serendeng (further separated into two tributaries: Paloh and Lassa), Belawai and Rajang. The Igan tributary is the major outlet for freshwater (Jiang et al., 2019). Mangroves are distributed in the brackish-water area in the southwestern estuary near the Rajang and Serendeng tributaries, and some freshwater trees, such as Casuarina, are observed in the northeastern and coastal areas (Scott, 1985). The thick coverage of vegetation, especially mangroves, in the Rajang estuary produces high-ash, high-sulfur, degraded sapric peats (Lampela et al., 2014). In the Rajang estuary, the tide is the diurnal to semidiurnal type and can extend to the city of Sibu (Staub et al., 2000; Staub and Gastaldo, 2003). The range of the tide increases from northeast (1.5 m) to southwest (2.5 m).
In the Rajang estuary, a substantial fraction of the surface sediment is composed of peat deposits with a maximum depth of 15 m (Staub and Gastaldo, 2003). The Rajang riverine freshwater drains the mineral soil, so the mean grain sizes of the sediment are much coarser than those of the Rajang estuary, where peatland is dominant in the delta region (Wu et al., 2019). Sediments in the Rajang estuary are composed of gley soils, podzol soils and alluvia soils (Staub and Gastaldo, 2003). Gley consists of mixed-layer illite–smectite, illite, kaolinite and minor amounts of chlorite. Gley is frequently observed in the central and southwestern parts of the estuary (Staub and Gastaldo, 2003). Podzols are dominant in gray–white to white clay, which is composed of kaolinite and illite. Podzols are found in some low-lying areas and in the landward part of the Rajang estuary (Staub and Gastaldo, 2003). Alluvial soils, which consist of illite, smectite and kaolinite, are found in the landward part of the coastal area of the estuary (Staub and Gastaldo, 2003). The input of total suspended solids from the Rajang River is up to 30 Mt yr−1 (Staub and Gastaldo, 2003).
Three small blackwater rivers, namely the Maludam, Simunjan and Sebuyau rivers, are characterized by their tea color, acidity and oxygen deficits (Kselik and Liong, 2004). The Maludam River, mainly located in Maludam National Park (the second-largest park in Sarawak), is a pristine river with minor human influences. The peat thickness in the riverbed reaches 10 m (Müller et al., 2015). The catchment of the Maludam River is 91.4 km2, and its average discharge is 4.4±0.6 m3 s−1 (Müller et al., 2015). However, the other two blackwater rivers have been undergoing severe disturbances due to human activities, mostly from plantations of commercial crops, such as oil palm and sago, as shown in Fig. 1d (Wetlands International, 2010). The grain size of sediments in blackwater rivers is much lower and receives more woody material than that of the Rajang River (Wu et al., 2019).
2.2 Sample collection and process
The water sampling stations are outlined in Fig. 1. The data collection surveys of the Rajang River and Rajang estuary were conducted in August 2016 (the dry season) and March 2017 (the wet season). Each data collection survey lasted 4 to 5 d, covering both flooding tides and ebbing tides. The water samples included freshwater from rivers, brackish water from different river tributaries and coastal saline water. In the Rajang watershed, the selection of water sampling stations depended on the salinity gradient, anthropogenic activities and water depth. In March 2017, we failed to collect samples upstream of the Rajang River in addition to saline samples from the Igan tributary, mainly due to the shallow water depth and strong current occurring at the time of collection. However, the three aforementioned blackwater rivers were included in the cruises. During the two cruises, surface water samples were collected using a pole sampler. The front of the sampler was attached to a 1 L high-density polyethylene bottle (Nalgene, USA). The length of the pole was 3–4 m to avoid contamination from the ship. The bottom water samples were collected using a precleaned 5 L Teflon-coated Niskin-X bottle that was hung on a nylon rope. Due to the limited sampling time and conditions, only three bottom water samples were collected in August 2016 and one bottom water sample was collected in March 2017. Water samples were filtered through acid-cleaned 0.4 µm pore size polycarbonate membrane filters (Whatman, UK) into a polyethylene bottle (Nalgene); then, the samples were frozen at −20∘ and packed into triple bags. The samples were thawed at room temperature in the clean laboratory and were acidified with ultrapure HCl to pH 1.7 in an ultraclean lab to transform and preserve metallic Fe in a soluble inorganic form (Lee et al., 2011). All the bottles used in sample collection and storage were prepared in a clean laboratory: the bottles were rinsed with Milli-Q water, immersed in 2 % Citranox detergent for 24 h, rewashed 5–7 times with Milli-Q water, leached in 10 % HCl for 7 d, rinsed 5–7 times with Milli-Q water again, filled with 0.06 mol L−1 ultrapure HCl, allowed to sit for 2 d at 60 ∘C and sealed in plastic bags.
2.3 Sample analyses
The dFe concentration was preprocessed using a single-batch resin extraction and the isotope dilution method. The acidified samples were preprocessed by a single-batch nitrilotriacetate-type (NTA-type) chelating resin (Qiagen, Valencia). Dissolved Fe can be quantitatively recovered after the oxidization of Fe2+ to Fe3+ by the addition of H2O2 (Lee et al., 2011). Here, dFe was quantified on a multicollector inductively coupled plasma mass spectrometer in the high-resolution mode (Neptune, Thermo Scientific, USA). The inlet system contained an Apex IR desolvator with a perfluoroalkoxy microconcentric nebulizer (ESI, USA) at a solution uptake rate of 50 µL min−1. All the tubes used for the analyses were acid-leached with 10 % HCl for 2 d at 60∘, rinsed five times with Milli-Q water, subsequently filled with 0.06 mol L−1 of ultrapure HCl under a class 100 clean flow bench and leached for another 2 d at 60∘. The analytical procedural blank and detection limit (3 times the standard deviation of the procedural blank) were both 0.06 nmol L−1. The accuracy of the method was tested by analyzing intercalibration samples, including one open-ocean SAFe D1 sample and one estuary water SLEW-3 sample (NRC, Canada). The measured dFe concentrations of the SAFe D1 and SLEW-3 samples were 0.66±0.05 and 10.0±0.4 nmol L−1, respectively, compared to the consensus values of 0.70±0.03 and 10.2±1.2 nmol L−1, respectively (Zhang et al., 2015).
During the field investigation, the salinity, temperature, pH and dissolved oxygen (DO) concentrations were detected in situ with a probe (AP-2000, Aquaread, UK). In the Rajang River, suspended particulate matter (SPM) samples were collected with precombusted 0.7 µm pore size Whatman GF/F filters, and the SPM concentration was calculated according to the weight difference of the filters before and after filtration. Dissolved organic carbon (DOC) samples were collected by filtering samples through 0.2 µm pore size nylon filters. For the samples collected in August 2016, the DOC concentrations were determined via an Aurora 1030W total organic carbon analyzer at the Center for Coastal Biogeochemistry at Southern Cross University (Lismore, Australia). The reproducibility of the concentrations was ±0.2 mg L−1. For the samples collected in March 2017, the DOC concentrations were determined by the high-temperature catalytic oxidation method with a total organic carbon analyzer (Shimadzu, Japan) at the State Key Laboratory of Estuarine and Coastal Research at East China Normal University (Shanghai, China), and the coefficient of variation was 2 % (Wu et al., 2013).
2.4 Calculation of dFe flux and yield
To estimate the magnitude of dFe flux from tropical rivers to coastal water, the following equation was used:
where Q is the dFe flux, C is the mean dFe concentration at the freshwater endmember (S<1), V is the river discharge and RF is the removal factor, which is based on the ratio of the integrated area of the dFe concentration to salinity to that of the line intercepts of the theoretical dilution (Hopwood et al., 2014). The riverine dFe yield is the ratio of dFe flux to the drainage area.
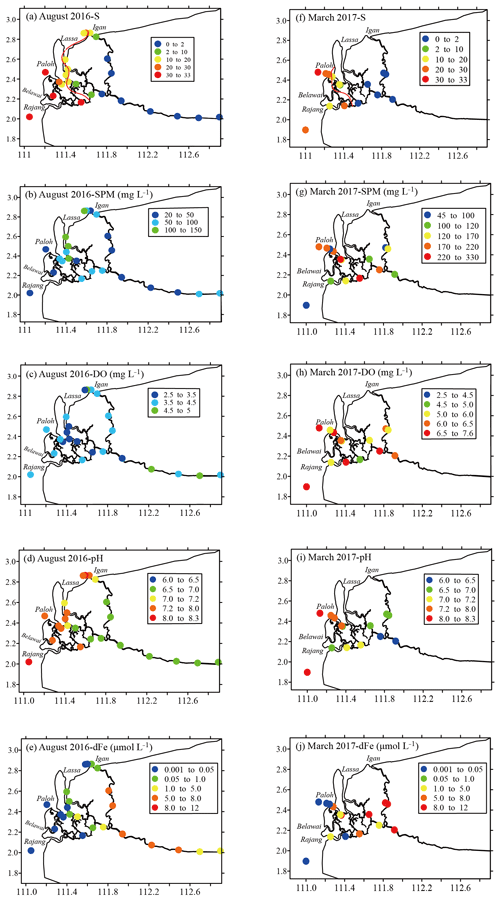
Figure 2Spatial distributions of the salinity (a and f), suspended particulate matter (SPM; b and g), dissolved oxygen (DO; c and h), pH (d and i) and dissolved iron (dFe; e and i) in the Rajang River in August 2016 and March 2017, respectively. The red solid line is the isosalinity line (S=15) linearly interpolated from S in this region.
3.1 Hydrographic properties in the Rajang and blackwater rivers
In August 2016 (the dry season), the salinity of the Rajang water samples ranged from 0.0 to 32.0 PSU (practical salinity units) and increased in salinity from the city of Sibu to the coastal zone (Table 1). In March 2017 (the wet season), the salinity varied from 0.0 to 30.1 PSU (Table 1). The salinity also increased along the water flow pathway in the Rajang estuary, with the exception of the Rajang tributary. The SPM concentration ranged from 24.2 to 327.2 mg L−1 and decreased in concentration from freshwater to seawater, but the highest water turbidity varied among channels and seasons. In August 2016, the peak SPM concentration was observed near the river mouth in the Serendeng tributary but moved landward in other tributaries (Fig. 2b). In March 2017, the peak of SPM concentration was located in freshwater of the Rajang tributary. The DO content recorded in March 2017 (mean: 6.1±0.7 mg L−1) was higher than that recorded in August 2016 (mean: 3.8±0.6 mg L−1) and decreased along the transport pathway of the Rajang drainage basin (Fig. 2c). The distribution of the DO concentration in Rajang varied between the two seasons, and a high value was found in the western estuary in March 2017 (Fig. 2c, h). The pH of water in the Rajang samples increased along the salinity gradient, with mean values of 7.1±0.5 (August 2016) and 7.1±0.6 (March 2017). As outlined in Fig. 2d and i, the seasonal variation in pH was not significant.
In blackwater rivers, salinity ranged from 0 to 23.5 PSU in the Maludam River and from 0 to 13.6 PSU in the Sebuyau River. The samples from the Simunjan River only included freshwater. All three blackwater rivers were anoxic at the freshwater endmembers, with DO concentrations <2 mg L−1. The mixing that occurred between river water and ocean water markedly increased the DO concentration. Moreover, the pH measured in these blackwater rivers was relatively low, especially in the Maludam River (minimum 3.7). The distributions of these properties in blackwater rivers are outlined in Supplement 1.
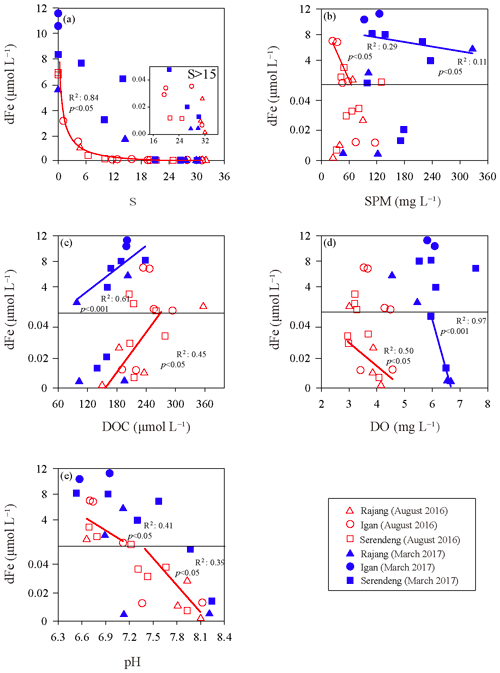
Figure 3Correlation of the dissolved iron (dFe) with the salinity (S) (a), suspended particulate matter (SPM) (b), dissolved organic carbon (DOC) (c), dissolved oxygen (DO) (d) and pH (e) in the Rajang estuary. The solid lines were the linear regressions between dFe and other factors, and the colors of the lines were coincident with the data points in different salinity range. Serendeng is the stations in tributary Paloh and Lassa, and Rajang is the stations in tributary Belawai and Rajang.
3.2 dFe in the Rajang River and estuary
The contour of dFe in Rajang surface water is shown in Fig. 2. We adopted Sibu as the separation location of the Rajang River and the Rajang estuary. The dFe concentrations in the Rajang River ranged from 3.3 to 7.3 µmol L−1 (mean: 5.5±1.7 µmol L−1) in August 2016 and varied from 4.2 to 8.3 µmol L−1 (mean: 6.4±2.9 µmol L−1) in March 2017. In the Rajang estuary, the dFe concentration ranged from 1.7 to 7.0 µmol L−1 (mean: 1.1±2.2 µmol L−1) and varied from 4.2 to 11.3 µmol L−1 (mean: 4.2±4.0 µmol L−1) in the dry season and the wet season, respectively. In both the Rajang River and the Rajang estuary, the concentration of dFe measured in the wet season was higher than that measured in the dry season.
The relationships between the dFe concentrations and other factors, such as salinity, SPM, DOC, DO and pH, in the Rajang estuary can be found in Fig. 3. The sites in Paloh and Lassa were combined with the Serendeng tributary, and the Belawai and Rajang tributaries were combined with the Rajang tributary. In the dry season, the dFe concentration exponentially decreased in low-salinity water (salinity <15), though we did not include the tidal influence. A linear relationship was found between dFe and SPM in the low-salinity area (R2=0.29, p<0.05). In the high-salinity area (S>15), dFe tended to be conservative (Fig. 3a) and displayed a linear relationship with the DOC concentration (), DO concentration () and pH (R2=0.39, p<0.05). In the wet season, the dFe concentration was higher in the Igan tributary than in the other branches. There was an intense addition of dFe that occurred between salinity of 5 and 15 PSU, mainly in the Serendeng tributary (Fig. 3a). Specifically, a linear correlation was found between dFe and SPM in the water samples when salinity was <15 PSU in the wet season (; Fig. 3b), especially in the Serendeng distributary. Moreover, a significant positive relationship was identified between dFe and the DOC concentration in the wet season in low-salinity waters (; Fig. 3c). The DO concentration was negatively correlated with dFe in the high-salinity area (), with a similar pattern observed in the dry season. The relationship between pH and dFe was not significant in the wet season.
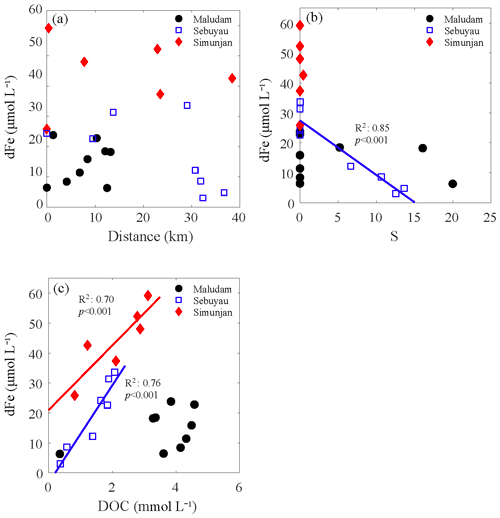
Figure 4Correlations among the distance (a), salinity (S) (b), dissolved organic carbon (DOC) (c) and dissolved iron (dFe) in blackwater rivers: Maludam, Sebuyau and Simunjan. The solid lines were the linear regressions between dFe and other factors, and the colors of the regression lines were coincident with the data points. We adopted the station at the upper stream as having distance of 0 and the downstream direction as positive.
3.3 dFe in blackwater rivers
The average dFe concentrations in the three blackwater rivers were 14.6±6.7 µmol L−1 (the Maludam River), 44.2±11.8 µmol L−1 (the Simunjan River) and 17.6±12.0 µmol L−1 (the Sebuyau River). The dFe concentration increased from the upper stream to the lower stream (Fig. 4a) but decreased during mixing. The distribution of dFe in blackwater rivers tended to be conservative in the Maludam and Sebuyau estuaries (Fig. 4b). Moreover, there were significantly positive correlations observed between dFe and the DOC concentration in the Sebuyau River and the Simunjan River (Fig. 4c), while the correlation between dFe and the DOC concentration in the Maludam River was weak due to an outlier in high-salinity water (S=20.0).
4.1 Seasonal and spatial variation in dFe in the Rajang River
In the dry season, the dFe concentration in the Rajang water (near the city of Sibu) ranged from 2.8 to 7.3 µmol L−1. In the wet season, the dFe concentration increased (Fig. 2). As precipitation is enhanced in the wet season, the strong water flow from the upper stream scours the watershed, delivering Fe-enriched terrestrial particles to the lower stream in the wet season (Meade et al., 1985; Taillefert et al., 2000). A large quantity of dFe may result from the dissolution of these particles originating from mechanical and chemical weathering, which leads to a significant addition of dFe in the wet season (Bhatia et al., 2013). Moreover, agricultural activities in the watershed, such as tillage, can result in rapid leaching in the wet season (Lehmann and Schroth, 2003; Tabachow et al., 2004). The changes in the soil structure likely enhanced soil erosion in the cropping land, especially in 2017 (the occurrence of La Niña events; Jiang et al. 2019). In addition, the changes in soil structure during agricultural activities can influence the exchange route of dissolved matter in vertical profiles; hence, a large proportion of dFe is likely to be transported during rainfall via water exchanges that occur (Haygarth et al., 2003; Johnes and Hodgkinson, 1998; Withers et al., 2001). The addition of dFe from cropland was also observed in many other study cases, such as the Krishna River drainage area (Kannan, 1984), the Palar and Cheyyār River basins (Rajmohan and Elango, 2005), and the Guadalquivir River (Lorite-Herrera and Jiménez-Espinosa, 2008). Eventually, stream-borne dFe was injected into the Rajang River via hydrological connections in the riparian ditches, causing dFe to be distributed to rivers from terrestrial runoff and flood discharges (Yan et al., 2016).
4.2 Seasonal and spatial variation in dFe in the Rajang Estuary
In the Rajang estuary, there was an intense removal of dFe when the water salinity was <15 PSU, especially in the dry season (Fig. 3a). This finding may be related to the flocculation of the negatively charged colloids with cations during the mixing of fresh-saline water. This phenomenon has been observed in many rivers and simulation experiments (Boyle et al., 1977; Oldham et al., 2017; Zhu et al., 2018). In addition, dFe was negatively correlated with SPM in low-salinity waters (Fig. 3b), indicating that dFe removal may also be linked to the absorption of SPM, as described in other studies (Van Beusekom and Jonge, 1994; Homoky et al., 2012; Zhang et al., 1995). However, there was an exceptionally high dFe concentration in samples with salinity from 5 to 15 PSU in the Serendeng tributary in the wet season. On the one hand, this high dFe concentration may be the result of peatland soils in the adjacent area because peatland soils host abundant dFe and organic ligands, and these organic compounds can enhance the solubility of Fe during transport (Krachler et al., 2010; Oldham et al., 2017; Shuhaimiothman, 2009). On the other hand, there could be other processes for dFe addition in the Rajang estuary, such as the desorption of SPM-bounded Fe to river water.
The balance between the adsorption and desorption of trace metal ions onto and from SPM, respectively, is complicated. These two processes could occur simultaneously and be influenced by different environmental conditions, such as the SPM content, pH, salinity, and adsorption strength between ions and SPM (Hatje et al., 2003; Jiann et al., 2013; Zhang et al., 2008). It has been confirmed that the partition coefficient of dFe decreases with increasing SPM concentration and is inversely proportional to the log of the SPM concentration, termed the particle concentration effect (Benoit, 1995; Jiann et al., 2013; Turner and Millward, 2002). Furthermore, Zhu et al. (2018) suggested that desorption from particles was the main reason for the dFe enhancement occurring in the river mouth area of the Changjiang estuary. In the wet season, Serendeng tributary samples were collected during a spring tide. In addition, the intensive plantation and agricultural activities in the Serendeng tributary modified the soil structure and leached a considerable amount of SPM at the flood tide. In Fig. 3a, a strong increase in the dFe and SPM contents at salinities of 5–15 PSU are shown. Given a similar level of SPM content among the Rajang, river waters of Texas (Jiann et al., 2013) and Changjiang estuary (Zhu et al., 2018), we assumed that the dFe enrichment under this special condition may be related to desorption from the riverine SPM, though we lacked experimental confirmation, e.g., a mixing experiment to validate our assumptions. In addition, the limited number of bottom water samples studied in the Rajang estuary also revealed that the addition of dFe from salinities of 5–15 PSU in the wet season might also be the result of the resuspension of bottom water sediments because the bottom water dFe concentration was much higher than the surface dFe concentration.
In the high-salinity zone (S>15 PSU), dFe tended to be conservative. The positive relationship observed between dFe and DOC in the dry season (Fig. 3c) may be a mirror of the chemical association between dFe and organic matter. Specifically, the combination of dFe and organic matter, especially pelagic organic matter, can resist salt-induced aggregation and lead to an input of bioavailable dFe to the coastal zone (Breitbarth et al., 2009; Krachler et al., 2005; Stolpe and Hassellov, 2007).
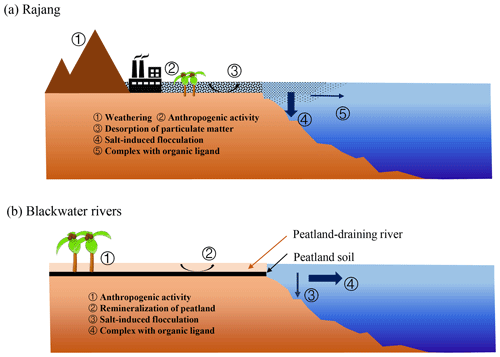
The multiple-linear-regression-analysis results of dFe and environmental factors, including salinity, SPM, DOC, DO and pH (the dry season: ; the wet season: ), revealed the observed pattern and explanations for more parameters. These results show that salinity and SPM were the main factors for the distribution of dFe in the Rajang estuary (p<0.05). The correlation between dFe and pH was limited in the wet season, suggesting a minor impact of pH on dFe. However, in the dry season, the dFe concentration was negatively correlated with pH (Fig. 3e) because Fe-enriched sediments can be acidified and mineralized by inorganic acids (H2CO3, HNO3 and H2SO3) and organic acids (oxalic acid, citric acid and siderophore) derived from chemical weathering and biological processes (Banfield et al., 1999; Lerman et al., 2007). The biogeochemical behavior of dFe in the Rajang River and estuary that we discussed above is summarized and conceptualized in Fig. 5a.
4.3 dFe in blackwater rivers
In blackwater rivers, dFe accumulated from the upper stream to the downstream before mixing. In the mixing zone, high concentrations of dFe were rapidly diluted (Fig. 4b). As evidenced by the water color, these peatland-draining rivers are characterized by extremely high levels of terrigenous DOM (Martin et al., 2018; Zhou et al., 2018). Given such high concentrations of DOM and the positive correlation between dFe and DOC (Fig. 4c), peatland should be a strong source of dFe. Consequently, the gradual enrichment of dFe along the river pathways was observed. Compared with the Maludam River, i.e., the drainage from an undisturbed peatland, the dFe concentrations in the Sebuyau River and the Simunjan River were significantly higher (Table 1). The difference in the dFe concentrations among the three blackwater rivers may result from the variation in environmental parameters around the drainage basin, especially the vegetation types and anthropogenic activities. Oil palm plantations covered a significant area in the watershed of the Sebuyau River and Simunjan River, as shown in Fig. 1d. To stimulate seedings in plantations, empty fruit bunches and oil palm mill effluent were returned to the cropland after oil extraction (Carron et al., 2015; Nelson et al., 2015). Intensive agricultural activities, such as tillage, further enhanced the decomposition of environmental parameters around the drainage basin, and these activities might improve the mechanical and chemical weathering that occurs in the plantation areas and increases the dFe concentration in the Sebuyau River and the Simunjan River, as discussed in Sect. 4.1.
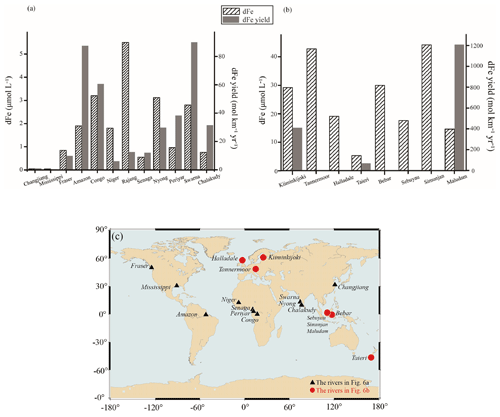
Figure 6Concentration and yield of dFe in large rivers (a) and blackwater rivers (b). The locations of the rivers are shown in (c). The concentration of dFe in the Rajang is the average of dFe in freshwater river section.
During the cruise, high-salinity samples were not obtained from the Maludam River or the Sebuyau River. For the samples with salinities ranging from 0 to 20.0 PSU, dFe removal was not significant, which is markedly different from the trend obtained in the Rajang estuary (Fig. 4b). The significant positive correlation observed between the dFe and DOC concentration revealed the tight connection between dFe and organic ligands in blackwater rivers (Fig. 4b). Recent studies have also noted that organic ligands originating from peatland enhance the iron-carrying capacity of river water (Krachler et al., 2005; Oldham et al., 2017). Approximately 20 % of dFe did not flocculate during a laboratory mixing experiment (Krachler et al., 2010). The biogeochemical behavior of dFe in blackwater rivers discussed above is summarized and conceptualized in Fig. 5b. Compared with the Rajang estuary, less dFe flocculated from the blackwater river estuaries due to complexing that occurred with organic matter, and the desorption of SPM was negligible during the mixing process. Remineralization of peatland soil is a great source of dFe in blackwater rivers, while the resuspension of sediment plays a critical role in the Rajang River system.
Table 2Concentration of dFe and the removal factor (RF) in some rivers.
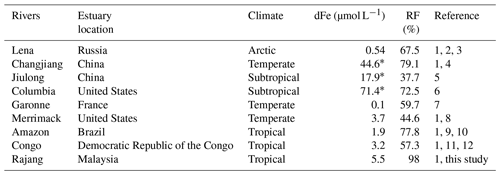
(1) Milliman and Farnsworth (2011). (2) Martin et al. (1993). (3) Guieu et al. (1996). (4) Zhu et al. (2018). (5) Zhang (1995). (6) Bruland et al. (2008). (7) Lemaire et al. (2006). (8) Boyle et al. (1974). (9) Aucour et al. (2003). (10) Moreira-Turcq et al. (2003). (11) Dupré et al. (1996). (12) Coynel et al. (2005). RF is the ratio of the integration of dFe concentration to salinity and the product of theoretical dilution line intercepts (Hopwood et al., 2014). ∗ In nanomoles per liter.
4.4 dFe flux and yield in tropical rivers
For the Rajang River, the mean concentration of the river endmember of the two seasons was 5.5±2.0 µmol L−1 and the mean removal factor was 98.0±0.6 %. The removal factor of dFe varied at the global scale. The Rajang RF is dominant among the recent results (Table 2). Coupled with the discharge rate (approximately 3600 m3 s−1), the dFe flux from the Rajang River was estimated to be kg yr−1. Compared to the Rajang River, salt-induced flocculation in blackwater rivers was weak, leading to a more effective transport of riverine dFe to the coastal ocean (Fig. 5). For the Maludam River, the dFe concentration of the river endmember was 14.6±6.8 µmol L−1 and RF = 0 due to the conservative dFe. The dFe flux in the Maludam River was approximately kg yr−1, produced from 432 km2 of peatland in Maludam National Park. This value was the same magnitude as the Rajang River dFe flux, suggesting that the dFe input was considerable in blackwater rivers. Malaysia hosts a peatland area of approximately 25 889 km2, and the dFe flux can reach approximately kg yr−1 on the basis of the yield from the Maludam River. Consequently, blackwater rivers contribute 10 times greater amounts of dFe than that contributed by the Rajang River to the coastal zone in Malaysia, even though their discharges are small (Milliman and Farnsworth, 2011). This terrestrial dFe may play an important role in supporting primary productivity in the adjacent ocean (Breitbarth et al., 2009; Laglera and Berg, 2009).
The concentration and yield of dFe varied among tropical rivers, as shown in Fig. 6. Compared with subtropical rivers, such as the Changjiang River (Zhu et al., 2018) and the Mississippi River (Shiller, 1997; Stolpe et al., 2010), tropical rivers contribute a greater amount of dFe to coastal areas with higher dFe yields, such as the Amazon River (Aucour et al., 2003; Bergquist and Boyle, 2006) and the Congo River (Coynel et al., 2005; Dupré et al., 1996). For rivers that have a similar discharge rate and drainage area to the Rajang River, such as the Fraser River in Canada, the dFe yield is significantly lower than that derived from the Rajang River (Cameron et al., 1995). The high dFe concentration and yield in tropical rivers likely result from the intensive weathering and leaching of rocks and sediments as well as the decomposition of abundant plantations under high temperatures and heavy precipitation (Bergquist and Boyle, 2006; Fantle and Depaolo, 2004). Compared with other tropical rivers, such as the Amazon River and the Congo River, the dFe yield is lower in the Rajang River and may be related to the difference in plantation types (Aucour et al., 2003; Coynel et al., 2005; Dupré et al., 1996). The peatland soils in the Rajang estuary may contribute to the higher dFe yield, as the Niger River passes through a dry savanna (Picouet et al., 2002). In contrast to the Niger River, the Sanaga River drains a savanna rainforest area and contains considerable amounts of SPM compared to the Rajang River. The dFe yield is comparable with that of the Rajang River. For some small tropical rivers, such as the Swarna River (Tripti et al., 2013), the Nyong River (Olivié-Lauquet et al., 1999), the Periyar River (Maya et al., 2007) and the Chalakudy River (Maya et al., 2007), the dFe yields and DOC concentrations are higher compared to those of the Rajang River. In these small tropical rivers, the drainage basins were covered with sediment-enriched organic matter, which may be a great source of dFe flux.
In blackwater rivers, the dFe yields were much higher than the amounts obtained in the Rajang River. The thick peatland soils were likely to be the main reason for the high dFe concentration in the blackwater rivers, as previously reported for the Kiiminkijoki River (Heikkinen, 1990), the Tannermoor River (Krachler et al., 2005), the Halladale River (Krachler et al., 2010), the Bebar River (Gastaldo, 2010) and the Taieri River (Hunter, 1983; Fig. 6b). Human impacts, such as agricultural activities and plantations of oil palm trees, may also contribute to the bulk dFe flux to blackwater rivers.
In this study, dFe was investigated in peatland-draining rivers and estuaries in Sarawak, Malaysia. The conclusions are as follows.
There was a significant seasonal variation in the dFe concentration in the Rajang River with a higher dFe concentration observed in the wet season, which is likely due to the dissolution of particular iron from upstream weathering. dFe removal was intense in the low-salinity area (salinity <15 PSU) of the Rajang estuary due to salt-induced flocculation and adsorption onto the SPM. In contrast, dFe tended to be conservative in the high-salinity area (salinity > 15 PSU), which may be due to binding between dFe and organic matter. In addition, there were significant additions of dFe in some tributaries from the desorption of SPM and anthropogenic inputs.
The dFe concentration in the blackwater rivers was 3–10 times greater than the dFe concentration in the Rajang River, which was related to the contribution of peatland soil. Anthropogenic activities in the watershed also influenced the dFe concentrations in the blackwater rivers. In contrast to the patterns observed in the Rajang estuary, there was no remarkable dFe removal occurring in the blackwater river estuary.
The dFe yield in the blackwater rivers was much higher than the dFe yield in the Rajang River. This result indicated that the dFe flux in the blackwater rivers can be crucial for coastal zones in Malaysia.
This study improved the understanding of the dFe distribution in Rajang and confirmed its regional influence. In addition, we showed that the blackwater rivers had an extremely high yield of dFe. Furthermore, anthropogenic activities may have a critical impact on the concentration and distribution of dFe in these tropical rivers in Malaysia.
The datasets will be provided upon reasonable request by the corresponding author.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-1805-2020-supplement.
JZ, MM, YW, SJ and XZ designed the study. JZ, ZZ, EA, FJ and MM performed the sample collection and in situ measurements for the first cruise. SJ, KZ, AM, EA, FJ and MM performed samplings and in situ measurements for the second cruise. XZ, YW and MM completed laboratory analyses. All co-authors contributed to the interpretation and discussion of the results. XZ prepared the paper, with suggestions from all the co-authors.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Biogeochemical processes in highly dynamic peat-draining rivers and estuaries in Borneo”. It is not associated with a conference.
We would like to thank the Sarawak Forestry Department and Sarawak Biodiversity Centre for permission to conduct collaborative research in Sarawak waters under permit no. NPW.907.4.4 (Jld.14)-161, park permit no. WL83/2017 and permit no. SBC-RA-0097-MM. Thanks to Lukas Chin and the Sea-Wonder crew for their support during the cruises. Technical support by Patrick Martin and Gonzalo Carrasco at Nanyang Technological University during the cruises and Yun Xue, Shuo Jiang and Wanwan Cao at East China Normal University in the laboratory analysis are also gratefully acknowledged.
The present study was kindly funded by the National Natural Science Foundation of China (41476065), the MOHE FRGS 15 Grant (FRGS/1/2015/WAB08/SWIN/02/1), the SKLEC Open Research Fund (SKLEC-KF201610) and the Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B08022).
This paper was edited by Steven Bouillon and reviewed by two anonymous referees.
Aucour, A. M., Tao, F. X., Moreiraturcq, P., Seyler, P., and Sheppard, S.: The Amazon River: behaviour of metals (Fe, Al, Mn) and dissolved organic matter in the initial mixing at the Rio Negro/Solimões confluence, Chem. Geol., 197, 271–285, 2003.
Banfield, J. F., Barker, W. W., Welch, S. A., and Taunton, A.: Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere, P. Natl. Acad. Sci. USA, 96, 3404–3411, 1999.
Batchelli, S., Muller, F. L. L., Chang, K. C., and Lee, C. L.: Evidence for strong but dynamic iron-humic colloidal associations in humic-rich coastal waters, Environ. Sci. Technol., 44, 8485–8490, 2010.
Bergquist, B. A. and Boyle, E. A.: Iron isotopes in the Amazon River system: weathering and transport signatures, Earth Planet. Sc. Lett., 248, 54–68, 2006.
Benoit, G.: Evidence of the particle concentration effect for lead and other metals in fresh waters based on ultraclean technique analyses, Geochim. Cosmochim. Ac., 59, 2677–2687, 1995.
Bhatia, M. P., Kujawinski, E. B., Das, S. B., Breier, C. F., Henderson, P. B., and Charette, M. A.: Greenland meltwater as a significant and potentially bioavailable source of iron to the ocean, Nat. Geosci., 6, 274–278, 2013.
Boyd, P. W. and Ellwood, M. J.: The biogeochemical cycle of iron in the ocean, Nat. Geosci., 3, 675–682, 2010.
Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., Coale, K. H., Cullen, J. J., de Baar, H. J. W., and Follows, M.: Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions, Science, 315, 612–617, 2007.
Boyle, E., Collier, R., Dengler, A. T., Edmond, J. M., Ng, A. C., and Stallard, R. F.: On the chemical mass-balance in estuaries, Geochim. Cosmochim. Ac., 38, 1719–1728, 1974.
Boyle, E. A., Edmond, J. M., and Sholkovitz, E. R.: The mechanism of iron removal in estuaries, Geochim. Cosmochim. Ac., 41, 1313–1324, 1977.
Brand, L. E., Sunda, W. G., and Guillard, R. R. L.: Limitation of marine phytoplankton reproductive rates by zinc, manganese, and iron, Limnology & Oceanography, 28, 1182-1198, 1983.
Braungardt, C. B., Achterberg, E. P., Elbaz-Poulichet, F., and Morley, N. H.: Metal geochemistry in a mine-polluted estuarine system in Spain, Appl. Geochem., 18, 1757–1771, 2003.
Breitbarth, E., Gelting, J., Walve, J., Hoffmann, L. J., Turner, D. R., Hassellöv, M., and Ingri, J.: Dissolved iron (II) in the Baltic Sea surface water and implications for cyanobacterial bloom development, Biogeosciences, 6, 2397–2420, https://doi.org/10.5194/bg-6-2397-2009, 2009.
Bruland, K. W., Lohan, M. C., Aguilar-Islas, A. M., Smith, G. J., Sohst, B., and Baptista, A.: Factors influencing the chemistry of the near-field Columbia River plume: Nitrate, silicic acid, dissolved Fe, and dissolved Mn, J. Geophys. Res.-Oceans, 113, C00B02, doi:10.1029/2007JC004702, 2008.
Cameron, E. M., Hall, G. E. M., Veizer, J., and Krouse, H. R.: Isotopic and elemental hydrogeochemistry of a major river system: Fraser River, British Columbia, Canada, Chemical Geology, 122, 149–169, 1995.
Carron, M. P., Auriac, Q., Snoeck, D., Villenave, C., Blanchart, E., Ribeyre, F., Marichal, R., Darminto, M., and Caliman, J. P.: Spatial heterogeneity of soil quality around mature oil palms receiving mineral fertilization, Eur. J. Soil Biol., 66, 24–31, 2015.
Coynel, A., Seyler, P., Etcheber, H., Meybeck, M., and Orange, D.: Spatial and seasonal dynamics of total suspended sediment and organic carbon species in the Congo River, Global Biogeochem. Cy., 19, GB4019, doi:10.1029/2004GB002335, 2005.
de Baar, H. J. W., de Jong, J. T. M.: Distributions, sources and sinks of iron in seawater., in: The Biogeochemistry of Iron in Seawater, Vol 7. IUPAC Series on analytical and physical chemistry of environmental systems, edited by: Turner D. R., Hunter K. A., Wiley, New York, United States, 123-234, 2001.
Dommain, R., Couwenberg, J., and Joosten, H.: Development and carbon sequestration of tropical peat domes in South-east Asia: links to post-glacial sea-level changes and Holocene climate variability, Quat. Sci. Rev., 30, 999–1010, 2011.
Dupré, B., Gaillardet, J., Rousseau, D., and Allegre, C. J.: Major and trace elements of river-borne material: the Congo Basin, Geochim. Cosmochim. Ac., 60, 1301–1321, 1996.
Fantle, M. S. and Depaolo, D. J.: Iron isotopic fractionation during continental weathering, Earth Planet. Sc. Lett., 228, 547–562, 2004.
Müller, D., Warneke, T., Rixen, T., Müller, M., Jamahari, S., Denis, N., Mujahid, A., and Notholt, J.: Lateral carbon fluxes and CO2 outgassing from a tropical peat-draining river, Biogeosciences, 12, 5967–5979, https://doi.org/10.5194/bg-12-5967-2015, 2015.
Gaillardet, J., Dupre, B., Allegre, C. J., and Négrel, P.: Chemical and physical denudation in the Amazon River Basin, Chem. Geol., 142, 141–173, 1997.
Gastaldo, R. A.: Peat or no peat: Why do the Rajang and Mahakam Deltas differ? Int. J. Coal Geol., 83, 162–172, 2010.
Guieu, C., Huang, W. W., Martin, J. M., and Yong, Y. Y.: Outflow of trace metals into the Laptev Sea by the Lena River, Mar. Chem., 53, 255–267, 1996.
Hatje, V., Payne, T. E., Hill, D. M., McOrist, G., Birch, G. F., and Szymczak, R.: Kinetics of trace element uptake and release by particles in estuarine waters: effects of pH, salinity, and particle loading, Environ. Int., 29, 619–629, 2003.
Haygarth, P. M., Hepworth, L., and Jarvis, S. C.: Forms of phosphorus transfer in hydrological pathways from soil under grazed grassland, Eur. J. Soil Sci., 49, 65–72, 2003.
Heikkinen, K.: Seasonal changes in iron transport and nature of dissolved organic matter in a humic river in northern Finland, Earth Surf. Proc. Land., 15, 583–596, 1990.
Herzog, S. D., Persson, P., and Kritzberg, E. S.: Salinity effects on iron speciation in boreal river waters, Environ. Sci. Technol., 51, 9747–9755, 2017.
Homoky, W. B., Severmann, S., Mcmanus, J., Berelson, W. M., Riedel, T. E., Statham, P. J., and Mills, R. A.: Dissolved oxygen and suspended particles regulate the benthic flux of iron from continental margins, Mar. Chem., 134-, 59–70, 2012.
Hopwood, M. J., Statham, P. J., and Milani, A.: Dissolved Fe (II) in a river-estuary system rich in dissolved organic matter, Eastuar. Coast. Shelf S., 151, 1–9, 2014.
Hunter, K. A.: On the estuarine mixing of dissolved substances in relation to colloid stability and surface properties, Geochim. Cosmochim. Ac., 47, 467–473, 1983.
Jiang, S., Müller, M., Jin, J., Wu, Y., Zhu, K., Zhang, G., Mujahid, A., Rixen, T., Muhamad, M. F., Sia, E. S. A., Jang, F. H. A., and Zhang, J.: Dissolved inorganic nitrogen in a tropical estuary in Malaysia: transport and transformation, Biogeosciences, 16, 2821–2836, https://doi.org/10.5194/bg-16-2821-2019, 2019.
Jiann, K. T., Santschi, P. H., and Presley, B. J.: Relationships between geochemical parameters (pH, DOC, SPM, EDTA Concentrations) and trace metal (Cd, Co, Cu, Fe, Mn, Ni, Pb, Zn) concentrations in river waters of texas (USA), Aquat. Geochem., 19, 173–193, 2013.
Jickells, T. D., An, Z. S., Andersen, K. K., Baker, A. R., Bergametti, G., Brooks, N., Cao, J. J., Boyd, P. W., Duce, R. A., and Hunter, K. A.: Global iron connections between desert dust, ocean biogeochemistry, and climate, Science, 308, 67–71, 2005.
Johnes, P. J. and Hodgkinson, R. A.: Phosphorus loss from agricultural catchments: pathways and implications for management, Soil Use Manage., 14, 175–185, 1998.
Joosten, H.: Peatlands: guidance for climate change mitigation through conservation, rehabilitation and sustainable use, Food and Agriculture Organization of the United Nations and Wetlands International, 53–57, 2012.
Kannan, S.: Problems of iron deficiency in different crop plants in India: causative factors and control measures, J. Plant Nutr., 7, 187–200, 1984.
Krachler, R., Jirsa, F., and Ayromlou, S.: Factors influencing the dissolved iron input by river water to the open ocean, Biogeosciences, 2, 311-315, 2005.
Krachler, R., Krachler, R. F., Kammer, F. V. D., Süphandag, A., Jirsa, F., Ayromlou, S., Hofmann, T., and Keppler, B. K.: Relevance of peat-draining rivers for the riverine input of dissolved iron into the ocean, Sci. Total Environ., 408, 2402–2408, 2010.
Kselik, R. A. L. and Liong, T. Y.: Hydrology of the peat swamp in the Maludam National Park, Betong Division, Sarawak, Alterra, the Netherlands/Forest Department Sarawak and Sarawak Forestry Corporation, Malaysia, 25 pp., 2004.
Laglera, L. M. and van den Berg, C. M. G.: Evidence for geochemical control of iron by humic substances in seawater, Limnol. Oceanogr., 54, 610–619, 2009.
Lampela, M., Jauhiainen, J., and Vasander, H.: Surface peat structure and chemistry in a tropical peat swamp forest, Plant Soil, 382, 329–347, 2014.
Lee, J. M., Boyle, E. A., Echegoyen-Sanz, Y., Fitzsimmons, J. N., Zhang, R., and Kayser, R. A.: Analysis of trace metals (Cu, Cd, Pb, and Fe) in seawater using single batch nitrilotriacetate resin extraction and isotope dilution inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 686, 93–101, 2011.
Lehmann J., Schroth G.: Nutrient leaching, in: Trees, crops, and soil fertility: concepts and research methods, edited by: Schroth G., Sinclair E. L., Wallingford, CAB International, UK, 151–166, 2003.
Lemaire, E., Blanc, G., Schäfer, J., Coynel, A., and Etcheber, H.: Dissolved Trace Metal-Organic Complexes in the Lot-Garonne River System Determined Using the C18 Sep-Pak System, Aquat. Geochem., 12, 21–38, 2006.
Lerman, A., Wu, L., and Mackenzie, F. T.: CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance, Mar. Chem., 106, 326–350, 2007.
Lorite-Herrera, M. and Jiménez-Espinosa, R.: Impact of agricultural activity and geologic controls on groundwater quality of the alluvial aquifer of the Guadalquivir River (province of Jaén, Spain): a case study, Environ. Geol., 54, 1391–1402, 2008.
Martin, J. H.: Glacial-interglacial CO2 change: The Iron Hypothesis, Paleoceanogr. Paleocl., 5, 1–13, 1990.
Martin, J. M., Guan, D. M., Elbaz-Poulichet, F., Thomas, A. J., and Gordeev, V. V.: Preliminary assessment of the distributions of some trace elements (As, Cd, Cu, Fe, Ni, Pb and Zn) in a pristine aquatic environment: The Lena River estuary (Russia), Mar. Chem., 43, 185–199, 1993.
Martin, P., Cherukuru, N., Tan, A. S. Y., Sanwlani, N., Mujahid, A., and Müller, M.: Distribution and cycling of terrigenous dissolved organic carbon in peatland-draining rivers and coastal waters of Sarawak, Borneo, Biogeosciences, 15, 6847–6865, https://doi.org/10.5194/bg-15-6847-2018, 2018.
Maya, K., Babu, K. N., Padmalal, D., and Seralathan, P.: Hydrochemistry and dissolved nutrient flux of two small catchment rivers, south-western India, Chem. Ecol., 23, 13–27, 2007.
Meade, R. H., Dunne, T., Richey, J. E., Santos, U. D. M., and Salati, E.: Storage and remobilization of suspended sediment in the lower amazon river of brazil, Science, 228, 488–490, 1985.
Miettinen, J., Shi, C., and Liew, S. C.: Two decades of destruction in Southeast Asia's peat swamp forests, Front. Ecol. Environ., 10, 124–128, 2012.
Milliman, J. D. and Farnsworth, K. L.: River discharge to the coastal ocean: runoff, erosion, and delivery to the coastal ocean, 298–301, https://doi.org/10.1017/cbo9780511781247, 2011.
Morillo, J., Usero, J., and Gracia, I.: Biomonitoring of trace metals in a mine-polluted estuarine system (Spain), Chemosphere, 58, 1421–1430, 2005.
Moore, C. M., Mills, M. M., Achterberg, E. P., Geider, R. J., Laroche, J., Lucas, M. I., Mcdonagh, E. L., Pan, X., Poulton, A. J., and Rijkenberg, M. J. A.: Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability, Nat. Geosci., 2, 867–871, 2009.
Moore, S., Evans, C. D., Page, S. E., Garnett, M. H., Jones, T. G., Freeman, C., Hooijer, A., Wiltshire, A. J., Limin, S. H., and Gauci, V.: Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes, Nature, 493, 660–663, 2013.
Moreira-Turcq, P., Seyler, P., Guyot, J. L., and Etcheber, H.: Exportation of organic carbon from the Amazon River and its main tributaries, Hydrol. Proc., 17, 1329–1344, 2003.
Morley, R. J. and Flenley, J. R.: Tectonic and climatic controls on the distribution and quality of Cretaceous coals, Oxford University Press, Oxford, U.K., 50–59, 1987.
Müller, D., Warneke, T., Rixen, T., Müller, M., Jamahari, S., Denis, N., Mujahid, A., and Notholt, J.: Lateral carbon fluxes and CO2 outgassing from a tropical peat-draining river, Biogeosciences, 12, 5967–5979, https://doi.org/10.5194/bg-12-5967-2015, 2015.
Mutalib, A. A., Lim, J. S., Wong, M. H., and Koonvai, L.: Characterization, distribution and utilization of peat in Malaysia, in: Proceedings of the International Sympsium on Tropical Peatland, edited by: Aminuddin, B. Y., Kuching, Malaysian Agricultural Research and Development Institute, Kuala Lumpur, 7–16, 1992.
Nelson, P. N., Banabas, M., Goodrick, I., Webb, M. J., Huth, N. I., and O'Grady, D.: Soil sampling in oil palm plantations: a practical design that accounts for lateral variability at the tree scale, Plant Soil, 394, 421–429, 2015.
Oldham, V. E., Miller, M. T., Jensen, L. T., and Luther III, G. W.: Revisiting Mn and Fe removal in humic rich estuaries, Geochim. Cosmochim. Ac., 209, 267–283, 2017.
Olivié-Lauquet, G., Allard, T., Benedetti, M. F., and Muller, J. P.: Chemical distribution of trivalent iron in riverine material from a tropical ecosystem: a quantitative EPR study, Water Res., 33, 2726–2734, 1999.
Picouet, C., Dupré, B., Orange, D., and Valladon, M.: Major and trace element geochemistry in the upper Niger River (Mali): physical and chemical weathering rates and CO2 consumption, Chem. Geol., 185, 93–124, 2002.
Rajmohan, N. and Elango, L.: Distribution of Iron, Manganese, Zinc and Atrazine in Groundwater in Parts of Palar and Cheyyar River Basins, South India, Environ. Monit. and Assess., 107, 115–131, 2005.
Raven, J. A.: The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources, New Phytol., 109, 279–287, 1988.
Saitoh, Y., Kuma, K., Isoda, Y., Kuroda, H., Matsuura, H., Wagawa, T., Takata, H., Kobayashi, N., Nagao, S., and Nakatsuka, T.: Processes influencing iron distribution in the coastal waters of the Tsugaru Strait, Japan, J. Oceanogr., 64, 815–830, 2008.
Sanders, C. J., Santos, I. R., Maher, D. T., Sadat-Noori, M., Schnetger, B., and Brumsack, H. J.: Dissolved iron exports from an estuary surrounded by coastal wetlands: Can small estuaries be a significant source of Fe to the ocean, Mar. Chem., 176, 75–82, 2015.
Scott, I. M.: Soils of the Central Sarawak Lowlands, East Malaysia: Kuching, Department of Agriculture, Sarawak, East Malaysia, 2302, 1985.
Shiller, A. M.: Dissolved trace elements in the Mississippi River: Seasonal, interannual, and decadal variability, Geochim. Cosmochim. Ac., 61, 4321–4330, 1997.
Shuhaimi-Othman, M., Ahmad, A. K., and Lim, E. C.: Metals concentration in water and sediment of Bebar peat swampy forest river, Malaysia, J. Biol. Sci., 9, 730–737, 2009.
Siong, V. L. E.: Determination of heavy metals in water and sediment of Rajang River at Pelagus Area, thesis, Faculty of Resource Science and Technology, University Malaysia Sarawak, Malaysia, 12 pp., 2015.
Staub, J. R., Among, H. L., and Gastaldo, R. A.: Seasonal sediment transport and deposition in the Rajang River delta, Sarawak, East Malaysia, Sediment. Geol., 133, 249–264, 2000.
Staub, J. R. and Esterle, J. S.: Provenance and sediment dispersal in the Rajang River delta/coastal plain system, Sarawak, East Malaysia, Sediment. Geol., 85, 191–201, 1993.
Staub, J. R. and Gastaldo, R.: Late quaternary sedimentation and peat development in the Rajang river delta, Sarawak, east Malaysia, in: Tropical deltas of Southeast Asia, edited by: Sidi, F. H., Nummedal, D., Imbert, P., Darman, H., and Posamentier, H. W., Society for Sedimentary Geology Special Publication, Tulsa, 100 Oklahoma, U.S.A., 71–78, 2003.
Stolpe, B. and Hassellöv, M.: Changes in size distribution of fresh water nanoscale colloidal matter and associated elements on mixing with seawater, Geochim. Cosmochim. Ac., 71, 3292–3301, 2007.
Stolpe, B., Guo, L., Alan, M. S., and Hassellöv, M.: Size and composition of colloidal organic matter and trace elements in the Mississippi River, Pearl River and the northern Gulf of Mexico, as characterized by flow field-flow fractionation, Mar. Chem., 118, 119–128, 2010.
Tabachow, R. M., Peirce, J. J., and Richter, D. D.: Biogeochemical Models Relating Soil Nitrogen Losses to Plant-Available N, Environ. Eng. Sci., 18, 81–89, 2004.
Tagliabue, A., Bowie, A. R., Boyd, P. W., Buck, K. N., Johnson, K. S., and Saito, M. A.: The integral role of iron in ocean biogeochemistry, Nature, 543, 51–59, 2017.
Taillefert, M., Bono, A. B., and Luther, G. W.: Reactivity of freshly formed Fe (III) in synthetic solutions and pore waters: voltammetric evidence of an aging process, Environ. Sci. Technol., 34, 2169–2177, 2000.
Tawan, A. S., Ling, T. Y., Nyanti, L., Sim, S. F., Grinang, J., Soo, C. L., Lee, K. S. P., and Ganyai, T.: Assessment of water quality and pollutant loading of the Rajang River and its tributaries at Pelagus area subjected to seasonal variation and river regulation, Environ. Dev. Sustain., 1–24, https://doi.org/10.1007/s10668-019-00374-9, 2019.
Trade Chakra: available at: http://www.tradechakra.com/economy/malaysia/industry-in-malaysia-179.php (last access: 20 June 2018), 2009.
Tripti, M., Gurumurthy, G. P., Balakrishna, K., and Chadaga, M. D.: Dissolved trace element biogeochemistry of a tropical river, Southwestern India, Environ. Sci. Pollut. Res., 20, 4067–4077, 2013.
Turner, A. and Millward, G. E.: Suspended particles: their role in estuarine biogeochemical cycles, Estuar. Coast. Shelf S., 55, 857–883, 2002.
Van Beusekom, J. E. E. and de Jonge, V. N.: The role of suspended matter in the distribution of dissolved inorganic phosphate, iron and aluminum in the Ems estuary, Neth. J. Aquat. Ecol., 28, 383–395, 1994.
Wetlands International: A quick scan of peatlands in Malaysia, wetlands international, Petaling Jaya, Malaysia, 50 pp., 2010.
Williams, R. J. P.: The Bakerian Lecture, 1981: Natural Selection of the Chemical Elements, P. R. Soc. London, 213, 361–397, 1981.
Withers, P. J. A., Edwards, A. C., and Foy, R. H.: Phosphorus cycling in UK agriculture and implications for phosphorus loss from soil, Soil Use Manage., 17, 139–149, 2001.
Wu, Y., Bao, H. Y., Unger, D., Herbeck, L. S., Zhu, Z. Y., Zhang, J., and Jennerjahn, T. C.: Biogeochemical behavior of organic carbon in a small tropical river and estuary, Hainan, China, Cont. Shelf Res., 57, 32–43, 2013.
Wu, Y., Zhu, K., Zhang, J., Müller, M., Jiang, S., Mujahid, A., Muhamad, M. F., and Sia, E. S. A.: Distribution and degradation of terrestrial organic matter in the sediments of peat-draining rivers, Sarawak, Malaysian Borneo, Biogeosciences, 16, 4517–4533, https://doi.org/10.5194/bg-16-4517-2019, 2019.
Xue, Y., Xia, H., Christie, P., Zhang, Z., Li, L., and Tang, C.: Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: a critical review, Ann. Botany, 117, 363–377, 2016.
Yan, B., Guan, J., Shesterkin, V., and Zhu, H.: Variations of dissolved iron in the Amur River during an extreme flood event in 2013, Chinese Geogr. Sci., 26, 679–686, 2016.
Zhang, J.: Geochemistry of trace metals from Chinese river/estuary systems: an overview, Estuar. Coast. Shelf S., 41, 631–658, 1995.
Zhang, R., John, S. G., Zhang, J., Ren, J., Wu, Y., Zhu, Z., Liu, S., Zhu, X., Marsay, C. M., and Wenger, F.: Transport and reaction of iron and iron stable isotopes in glacial meltwaters on Svalbard near Kongsfjorden: From rivers to estuary to ocean, Earth Planet. Sci. Lett., 424, 201–211, 2015.
Zhang, Y. Y., Zhang, E. R., and Zhang, J.: Modeling on adsorption–desorption of trace metals to suspended particle matter in the Changjiang Estuary, Environ. Geol., 53, 1751–1766, 2008.
Zhou, Y., Martin, P., and Müller, M.: Composition and cycling of dissolved organic matter from tropical peatlands of coastal Sarawak, Borneo, revealed by fluorescence spectroscopy and parallel factor analysis, Biogeosciences, 16, 2733–2749, https://doi.org/10.5194/bg-16-2733-2019, 2019.
Zhu, X., Zhang, R., Wu, Y., Zhu, J., Bao, D., and Zhang, J.: The remobilization and removal of Fe in estuary – a case study in the Changjiang Estuary, China, J. Geophys. Res.-Oceans, 123, 2539–2553, 2018.