the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Evaluating the response of δ13C in Haloxylon ammodendron, a dominant C4 species in Asian desert ecosystems, to water and nitrogen addition as well as the availability of its δ13C as an indicator of water use efficiency
Zixun Chen
Xuejun Liu
Xiaoqing Cui
Yaowen Han
Guoan Wang
Jiazhu Li
Variations in precipitation and atmospheric N deposition affect water and N availability in desert and thus may have significant effects on desert ecosystems. Haloxylon ammodendron is a dominant plant in Asian desert, and addressing its physiological acclimatization to the changes in precipitation and N deposition can provide insight into how desert plants adapt to extreme environments by physiological adjustment. Carbon isotope ratio (δ13C) in plants has been suggested as a sensitive long-term indicator of physiological acclimatization. Therefore, this study evaluated the effect of precipitation change and increasing atmospheric N deposition on δ13C of H. ammodendron. Furthermore, H. ammodendron is a C4 plant; whether its δ13C can indicate water use efficiency (WUE) has not been addressed. In the present study, we designed a field experiment with a completely randomized factorial combination of N and water and measured δ13C and gas exchange of H. ammodendron. Then we calculated the degree of bundle-sheath leakiness (φ) and WUE of the assimilating branches of H. ammodendron. δ13C and φ remained stable under N and water supply, while N addition, water addition and their interaction affected gas exchange and WUE in H. ammodendron. In addition, δ13C had no correlation with WUE. These results were associated with the irrelevance between δ13C and the ratio of intercellular to ambient CO2 concentration (ci ca), which might be caused by a special value (0.37) of the degree of bundle-sheath leakiness (φ) or a lower activity of carbonic anhydrase (CA) of H. ammodendron. In conclusion, δ13C of H. ammodendron is not sensitive to global change in precipitation and atmospheric N deposition and cannot be used for indicating its WUE.
- Article
(989 KB) - Full-text XML
-
Supplement
(287 KB) - BibTeX
- EndNote
Recently, global precipitation pattern has changed significantly (Frank et al., 2015; Knapp et al., 2015), and atmospheric N deposition has continued to rise (Galloway et al., 2004; Liu et al., 2013; Song et al., 2017). Previous researchers have suggested that arid ecosystems are most sensitive to climate change (Reynolds et al., 2007; Huang et al., 2016), while global change in precipitation and atmospheric N deposition has an important impact on water and N availability in desert (Huang et al., 2018). Thus, these changes may have significant effects on desert ecosystems. Haloxylon ammodendron is a dominant species in desert regions, especially in Asia. Studying the physiological responses of H. ammodendron to global change can provide insight into how desert plants adapt to extreme environments by physiological adjustment. Carbon isotope ratio (δ13C) in plants depends on the ratio of intercellular to ambient CO2 concentration (), which reflects the balance between inward CO2 diffusion rate, regulated by stomatal conductance (gs), and CO2-assimilating rate (A) (Farquhar and Richards, 1984) and has been suggested as a sensitive long-term indicator of physiological acclimatization (Battipaglia et al., 2013; Cernusak et al., 2013; Tranan and Schubertt, 2016; Wang and Feng, 2012). Therefore, investigating the variations in δ13C of H. ammodendron under water and nitrogen addition can enhance understanding of physiological responses of desert plants to future changes in precipitation and atmospheric N deposition.
A large quantity of works have been devoted to the relationships between C3 plant δ13C and water availability or precipitation (e.g., Diefendorf et al., 2010; Kohn, 2010; Liu et al., 2005; Ma et al., 2012; Serret et al., 2018; Stewart et al., 1995; Wang et al., 2005, 2008) and nitrogen availability (e.g., Cernusak et al., 2007; Li et al., 2016; Sparks and Ehleringer, 1997; Yao et al., 2011; Zhang et al., 2015). However, a relatively small amount of research has focused on the responses of C4 plant δ13C to water availability or precipitation (Ellsworth et al., 2017; Liu et al., 2005; Rao et al., 2017; Wang et al., 2006) and nitrogen availability (Ma et al., 2016; Schmidt et al., 1993). For C4 plants, δ13C is controlled by both the ci ca ratio and the degree of bundle-sheath leakiness (φ), the proportion of CO2 produced within bundle-sheath cells from C4 acids that leaks back to mesophyll cells (Ellsworth and Cousins, 2016; Ellsworth et al., 2017; Farquhar, 1983). Thus, the responses of C4 plant δ13C to water and N availability are also affected by φ. Genetic factors control φ values, which causes the interspecific differences in δ13C, even the responses of plant δ13C to water and N availability (Gresset et al., 2014). On the other hand, enzymatic activity of carbonic anhydrase (CA) may influence δ13C in C4 plants (Cousins et al., 2006). CA is an enzyme that catalyzes the hydration of CO2 in mesophyll cells to form bicarbonate (HCO). Previous studies showed that CA activity in most C4 plants is usually low, just sufficient to support photosynthesis (Cousins et al., 2006; Gillon and Yakir, 2000, 2001; Hatch and Burnell, 1990). H. ammodendron is a typical C4 plant. How its δ13C responds to water and N availability has never been addressed.
Foliar δ13C in C3 plants has been considered as a useful indicator of intrinsic water use efficiency (WUE) (Farquhar, 1983). However, although some studies have suggested that δ13C of C4 plants could also indicate its WUE (Henderson et al., 1992; Wang et al., 2005; Cernusak et al., 2013; Ellsworth and Cousins, 2016), this statement is still controversial. The relationship between δ13C and WUE is based on the links between ci ca ratio and δ13C and between ci ca ratio and WUE (Ehleringer and Cerling, 1995). For C3 plants, δ13C always decreases with an increase in ci ca ratio; but for C4 plants, the correlation between δ13C and ci ca ratio depends on the φ value (Cernusak et al., 2013) and CA activity (Cousins et al., 2006). As mentioned above, φ value is under genetic control, and the CA activity changes across species (Cousins et al., 2006; Gillon and Yakir, 2000, 2001; Hatch and Burnell, 1990); thus, the correlation between δ13C and ci ca ratio, as well as the relationship between WUE and δ13C, shows interspecific difference. Whether δ13C of H. ammodendron indicates WUE has never been evaluated.
In this study, we designed an experiment with multiple water and nitrogen supplies in the southern Gurbantünggüt Desert in Xinjiang Uygur Autonomous Region, China. We measured the δ13C, gas exchange and WUE of the assimilating branches of H. ammodendron. We had two objectives. One objective was to evaluate the response of the dominant plant of Asian desert to future changes in precipitation and atmospheric N deposition by revealing the effects of water and N supply on δ13C of H. ammodendron. The other was to explore the availability of δ13C as the indicator of water use efficiency in H. ammodendron.
2.1 Definitions and basic equations
Stable carbon isotopic ratio (δ13C) of natural materials is expressed as
where (13C 12C)sample and (13C 12C)standard are the 13C 12C ratio of the sample and of the Pee Dee Belemnite (PDB) standard, respectively. Farquhar (1983) proposed the pattern of carbon isotopic discrimination (Δ) in C4 plants:
where δ13Cplant and δ13Cair are the δ13C values of plants and CO2 in the ambient air, respectively. The parameter a (= 4.4 ‰; Craig, 1954) is the carbon isotopic fractionation in the diffusion of CO2 into internal leaves; b4 (= −5.9 ‰; O'Leary, 1984) is the combined carbon isotopic fractionations occurring in the processes of gaseous CO2 dissolution, hydration–dehydration reactions of CO2 and HCO in mesophyll cells, and HCO carboxylation by PEP (phosphoenolpyruvate) carboxylase; s (= 1.8 ‰; O'Leary, 1984) is the carbon isotopic fractionation during diffusion of CO2 out of the bundle-sheath cells; and b (= 27 ‰; Farquhar and Richards, 1984) is the carbon isotopic fractionation of CO2 carboxylation by RuBP (ribulose-1,5-bisphosphate) carboxylase. The variable φ is the proportion of CO2 production within bundle-sheath cells from C4 acids that leaks back to mesophyll cells, and ci ca is the ratio of intercellular to ambient CO2 concentration. Eq. (2) can be transformed into the following format:
According to Eq. (3), if the coefficient [b4+φ (b−s) − a] is greater than 0, δ13C decreases with increasing ci ca; if this coefficient is lower than 0, δ13C increases with increasing ci ca.
Water use efficiency (WUE) is defined as the amount of assimilated carbon dioxide by plants under the consumption per unit of water. There are two characteristics of WUE, instantaneous WUE (ins-WUE) and intrinsic WUE (int-WUE). Ins-WUE can be calculated by
where A is photosynthetic rate, E is transpiration rate and v is calculated by
where ei and ea are the water vapor pressure inside and outside the leaves, and p is the atmospheric pressure.
The definition of int-WUE is
where gs is stomatal conductance.
2.2 Study site and species
This experiment was conducted at the Fukang Station of Desert Ecology, Chinese Academy of Sciences, on the southern edge of the Gurbantünggüt Desert (44∘26′ N, 87∘54′ E) in northwestern China. The altitude of the study site is 436.8 m above average sea level (a.s.l.). It is a typical continental arid, temperate climate, with a hot summer and cold winter in the area. The mean annual temperature is 7.1 ∘C, and the mean annual precipitation is 215.6 mm, with a potential evaporation of about 2000 mm. The mean annual temperature and the annual rainfall amount in the sampling year are 10.23 ∘C and 122.7 mm (Cui, 2018). The soil type is grey desert soils (Chinese classification) with aeolian sands on the surface (0–100 cm). The percentages of clay (< 0.005 mm), silt (0.005–0.063 mm), fine sand (0.063–0.25 mm) and medium sand (0.25–0.5 mm) range from 1.63 %–1.76 %, 13.79 %–14.15 %, 55.91 %–56.21 % and 20.65 %–23.23 %, respectively (Chen et al., 2007). The soil is highly alkaline (pH = 9.55 ± 0.14) with low fertility. The vegetation is dominated by Haloxylon ammodendron and Haloxylon persicum with about 30 % coverage. Herbs include ephemerals, annuals and small perennials, with a cover of ca. 40 % (Fan et al., 2013). Although the coverage of the two Haloxylon species is a little lower than that of herbs, the biomass of the former is much larger than that of the latter, because Haloxylon plants are shrubs with an average height of 1.5 m whereas the latter are very low herbaceous plants. Biological soil crusts are distributed widely on the soil between the herbs and Haloxylon, with almost 40 % coverage (Zhang et al., 2007).
The present study focused on Haloxylon ammodendron because it is the dominant species in Asian desert. H. ammodendron is a species of Chenopodiaceae, which is a xerophytic and halophytic woody plant (Cui et al., 2017). The leaves of H. ammodendron have been completely degraded due to the extreme drought, and the assimilation branches, which are the glossy green branches (Fig. S1), perform the same functions as the leaves. Due to its drought tolerance, H. ammodendron is widely distributed in desert areas.
2.3 Experimental design
A field experiment with a completely randomized factorial combination of water and nitrogen has been conducted from 2014 to 2017. We designed two water addition levels (0, 60 mm yr−1; W0, W1) based on the prediction that precipitation will increase by 30 % in northern China in the next 30 years (Liu et al., 2010), and three levels of N addition (0, 30, 60 kg N ha−1 yr−1; N0, N1 and N2), because N deposition has reached 35.4 kg N ha−1 yr−1 in the nearby city Ürümqi (Cui et al., 2017) and will double by 2050 relative to the early 1990s (Galloway et al., 2008). Therefore, there were six treatments (W0N0, W0N1, W0N2, W1N0, W1N1 and W1N2) in this experiment. Four replicates of each treatment were set, making a total of 24 plots with a size of 10 m × 10 m. A small sub-plot with a size of 1.5 m × 1.5 m was set in each plot. A well-grown H. ammodendron was enclosed in the center of the sub-plot. The average height and coverage of an individual H. ammodendron were 1.5 m and 1.9 m2, respectively, and did not vary significantly across the plots. The type of nitrogen used in the present study is NH4NO3. To simulate natural water and N inputs, the treatments were applied in equal amounts, 12 times, once a week in April, July and September, as 5 mm m−2 of water and 2.5 or 5 kg N ha−1 each week (Cui et al., 2017). Usually, water addition was with a sprinkler kettle, irrigating over the canopy of H. ammodendron.
2.4 Measurements of gas exchange and WUE
As mentioned above, the main assimilating organ of H. ammodendron is the assimilation branches. Thus, we conducted gas exchange measurements on the assimilation branches of the H. ammodendron grown in the sub-plots by a LI-6400 portable photosynthesis system. The measurements were conducted on 27–29 June 2016, which is the main growing season of H. ammodendron. It may be most appropriate to take measurements during this period, and the results of the measurements are therefore more representative. Previous studies have also usually conducted this measurement during the growing season (Nyongesah and Wang, 2013; Cui, 2018; Gong et al., 2019). The ins-WUE was calculated based on these measured gas exchange traits by Eq. (4) and int-WUE by Eq. (6). At each plot, the top assimilating branches of a mature individual were selected randomly for the measurement of gas exchange, which includes photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), the ambient CO2 concentration (ca) and the intercellular CO2 concentration (ci). Before the measurement of gas exchange, it takes about 5 s to stabilize after the assimilating branches were inserted in the cuvette. We repeated 10 times on the same assimilating branches for each measurement. Our measurements were carried out under the conditions of a standard 450 mmol mol−1 CO2 concentration at a flow rate of 500 mmol s−1 above saturation in photo flux density of 1600 mmol m−2 s−1. The temperature of the assimilating branches varied from 29.5 to 30.5 ∘C during the entire period of gas exchange measurements.
2.5 Sample collection
Sample collection was conducted in 20 July, during the addition of water and nitrogen. Considering that there is a considerable difference in δ13C between buds and young and matured leaves, we collected the mature assimilating branches of H. ammodendron for the δ13C measurements. All H. ammodendron individuals grown in plots (10 m × 10 m) were sampled. Eight pieces of the mature assimilating branches (15–20 cm long) were collected from each individual; two pieces of assimilating branches were collected at each of the four cardinal directions from the positions of full irradiance. All assimilating branches from the same plot were combined into one sample. After the samples were collected, they were immediately divided into two parts randomly and taken back to the laboratory at Fukang Station. The first part was used to determine the chlorophyll content. The second part was immediately inactivated in a 105 ∘C oven in the laboratory at Fukang Station and then brought back to Beijing in a ziplock bag. The time interval between sample collection and inactivation is very short. After inactivation, the carbon exchange of the assimilating branches stops, so the isotope composition of the samples will not change anymore. All plant samples of the second part were air-dried immediately in the laboratory in Beijing. Then the samples were ground into a fine powder using a steel ball mixer mill MM200 (Retsch GmbH, Haan, Germany) for the measurements of δ13C and N contents.
2.6 Measurements of plant δ13C, plant N and chlorophyll contents
The δ13C and N measurements were performed on a DeltaPlus XP mass spectrometer (Thermo Scientific, Bremen, Germany) coupled with an automated elemental analyzer (Flash EA1112, CE Instruments, Wigan, UK) in continuous-flow mode, at the Stable Isotope Laboratory of the College of Resources and Environmental Sciences, China Agricultural University. The carbon isotopic ratios were reported in the delta notation relative to the V-PDB standard. For this measurement, we obtained standard deviations lower than 0.15 ‰ for δ13C among replicate measurements of the same sample. And standard deviations for the N measurements were 0.1 %.
The chlorophyll contents of all samples were determined immediately when the samples were taken back in the laboratory at Fukang Station. The samples were first extracted by 95 % ethyl alcohol (0.5 g sample to 25 mL ethyl alcohol), and then the absorbency was measured under the wavelengths 665 and 649 mm by the spectrophotometer. The content of chlorophyll a and b was calculated by the following equations:
where OD665 and OD649 are the absorbency under the wave lengths of 665 and 649 mm, respectively.
2.7 Calculation of the degree of bundle-sheath leakiness
The degree of bundle-sheath leakiness (φ) was calculated by the transformation of Eq. (2):
In this equation, parameters a, b4, b and s are constant, while δ13Cplant and ci ca are the measured values of our samples. We did not measure the δ13Cair at our study site, so we had to use an approximation of the δ13Cair to do this ϕ calculation. The approximated value we used is −9.77 ‰, which was measured at Donglingshan, Beijing, north China, in September 2019. The two sites should have similar δ13Cair because the two sites are located in countryside with less human activity and have a similar distance from the nearest city. The straight line distances between Donglingshan and the city center of Beijing as well as between our study site and Ürümqi city are about 90 km. In addition, since the δ13Cair has large diurnal and seasonal variations, we used the published range of δ13Cair from May to July in Shangdianzi, China (data come from Global Monitoring Laboratory, Earth System Research Laboratories, https://www.esrl.noaa.gov/gmd, last access: 21 March 2021) to calculate the minimum and maximum δ13Cair of the time period, which is the main growing season for H. ammodendron. Finally, the δ13Cair used in the calculation ranged from −10.52 ‰ to −9.01 ‰ with an average of −9.77 ‰.
2.8 Statistical analysis
Statistical analyses were conducted using SPSS software (SPSS for Windows, Version 20.0, Chicago, IL, United States). One-way analysis of variance (ANOVA) and two-way analysis of variance (ANOVA) were used to compare the difference of δ13C and other physiological traits between each treatment. Pearson analysis was used to determine the correlation among δ13C, WUE and ci ca in H. ammodendron.
3.1 Plant δ13C under water and nitrogen addition
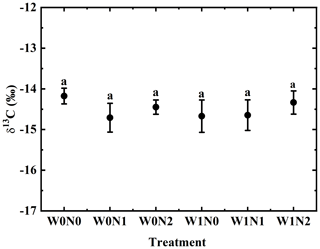
The δ13C of the assimilating branches of H. ammodendron in the six treatments W0N0, W0N1, W0N2, W1N0, W1N1 and W1N2 was −14.18 ± 0.19 ‰, −14.71 ± 0.35 ‰, −14.45 ± 0.18 ‰, −14.67 ± 0.40 ‰, −14.65 ± 0.38 ‰ and −14.344 ± 0.29 ‰. One-way ANOVA showed no significant variation in δ13C across treatments (p= 0.79, Fig. 1). Two-way ANOVA suggested that δ13C was not affected by water addition (p= 0.68), N addition (p= 0.61) or their interaction (p= 0.56, Table 1).
Table 1The p values of all measured and calculated indexes in plants under two-way ANOVA analysis of water (W) and nitrogen (N) additions.
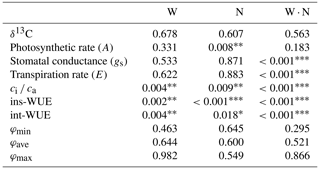
Note. φmin, φave and φmax
represent the φ values calculated from the minimum,
average and
maximum δ13Cair .*, and indicate a significant influence. W ⋅ N represents the interaction between water addition and N addition.
3.2 Gas exchange and WUE under water and nitrogen addition
Photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E) and ci ca ranged from 12.11 to 39.35 µmol CO2 m−2 s−1, from 0.09 to 0.31 mol H2O m−2 s−1, from 2.87 to 8.49 mmol H2O m−2 s−1 and from 0.11 to 0.57, respectively. One-way ANOVA showed significant changes in leaf gas exchange across the six treatments (p < 0.01 for A, gs, E and ci ca, Fig. 2). Two-way ANOVA suggested that water addition had exerted an effect on ci ca (p < 0.01), that N additions influenced A (p < 0.01) and ci ca (p= 0.009), and that the interaction between water and N supply played a role in gs (p < 0.001), E (p < 0.001) and ci ca (p < 0.001, Table 1).
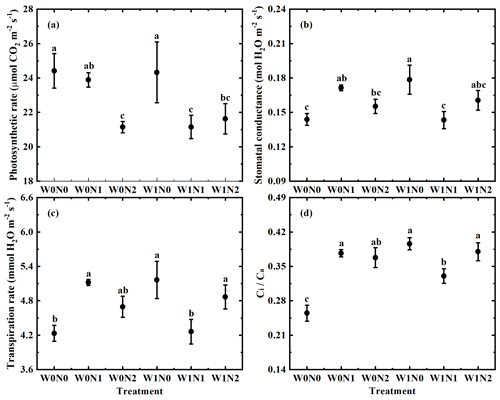
Figure 2Variations in photosynthetic rate (a), stomatal conductance (b), water use efficiency (c) and ci ca (d) across water (W) and nitrogen (N) additions. The spot represents the mean value of four replicates with error bars denoting the standard error (SE).
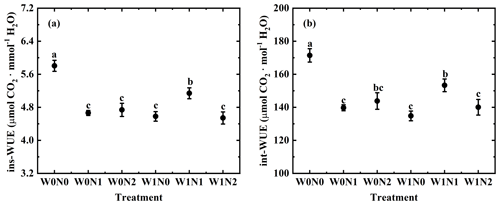
Instantaneous WUE (ins-WUE) and intrinsic WUE (int-WUE) ranged from 3.09 µmol CO2 mmol H2O to 8.49 µmol CO2 mmol H2O and from 93.64 µmol CO2 mol H2O to 208.47 µmol CO2 mmol H2O, respectively. One-way ANOVA showed significant changes in these two indexes across these treatments (both p < 0.001, Fig. 3). Two-way ANOVA suggested that water addition, N addition and their interaction all have a significant effect on these two indexes (all p < 0.05, Table 1).
3.3 Correlations among δ13C, WUE and ci ca ratio
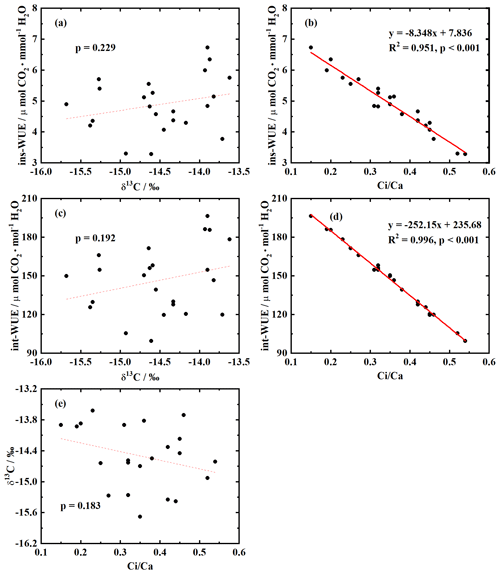
In order to test whether δ13C in H. ammodendron can indicate WUE, the relationships among δ13C, ins-WUE, int-WUE and ci ca ratio were revealed in this study. Our results showed no correlation between δ13C and ins-WUE (p= 0.23, Fig. 4a), between δ13C and int-WUE (p= 0.23, Fig. 4c), or between δ13C and ci ca ratio (p= 0.18, Fig. 4e). However, there was a negative correlation between ins-WUE and ci ca ratio (p < 0.001, Fig. 4b) and between int-WUE and ci ca ratio (p < 0.001, Fig. 4d).
3.4 The degree of bundle-sheath leakiness under water and nitrogen addition
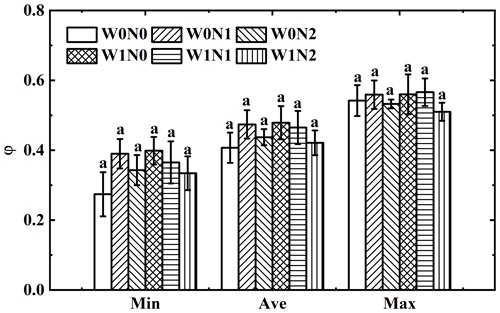
The φ value calculated from the minimum δ13Cair ranged from 0.16 to 0.50 with a mean value of 0.35; the φ value calculated from the maximum δ13Cair ranged from 0.44 to 0.70 with a mean value of 0.55, and the φ value calculated from the average δ13Cair ranged from 0.32 to 0.59 with a mean value of 0.45. One-way ANOVA showed no significant variation in φ calculated from the minimum, average and maximum δ13Cair across treatments (p= 0.60 for the φ calculated from the minimum δ13Cair, p= 0.77 for the φ calculated from the average δ13Cair and p= 0.90 for the φ calculated from the maximum δ13Cair, Fig. 5). Two-way ANOVA suggested that φ was not affected by water addition (p= 0.46 for the φ calculated from the minimum δ13Cair, p= 0.64 for the φ calculated from the average δ13Cair and p= 0.98 for the φ calculated from the maximum δ13Cair), N addition (p= 0.65 for the φ calculated from the minimum δ13Cair, p= 0.60 for the φ calculated from the average δ13Cair and p= 0.55 for the φ calculated from the maximum δ13Cair) or their interaction (p= 0.30 for the φ calculated from the minimum δ13Cair, p= 0.52 for the φ calculated from the average δ13Cair and p = 0.87 for the φ calculated from the maximum δ13Cair, Table 1).
The δ13C of the assimilating branches in H. ammodendron did not change across treatments (Fig. 1, Table 1), suggesting that neither water addition nor nitrogen addition influenced the δ13C of H. ammodendron. Previous studies also reported no significant relationship between δ13C of C4 plant and water availability (Swap et al., 2004; Wang et al., 2008) and between δ13C of C4 plant and nitrogen availability (Yao et al., 2011; Yang et al., 2017).
In general, the effects of water availability and nitrogen availability on δ13C are dependent on ci ca ratio, which reflects the balance between stomatal conductance (gs) and photosynthetic rate (A) (Farquhar and Richards, 1984). Stomatal conductance (gs) usually increases with increasing water availability under water addition. Although two-way ANOVA suggested that water addition had no effect on both A and gs (Table 1), one-way ANOVA showed that gs was higher in W1N0 than that in W0N0 (Fig. 2b), indicating that water addition had a positive effect on gs under ambient N conditions. Increasing gs under water supply will lead to the rise of intercellular CO2 because of the decrease in diffusional resistance to CO2. As a result, ci ca ratio was observed to increase with increasing moisture (Fig. 2d, Table 1). However, δ13C remained stable under water addition (Fig. 1, Table 1). Thus, ci ca ratio could not explain the observed response of δ13C to water supply.
For most plants in natural ecosystems, nitrogen is the key factor limiting plant growth (Hall et al., 2011). Thus, nitrogen addition usually causes plants to absorb more N. However, extreme drought could prevent plants from absorbing N even under high N supply. In the present experiment, N supply was found to have an effect on N contents in H. ammodendron. Relative to the control treatment (W0N0), N contents increased with N supply under low N addition but remained unchanged under high addition (Tables S1, S2). Nitrogen is the main constituent of Rubisco (ribulose – 1,5 – bisphosphate carboxylase oxygenase) and chlorophyll in plants. Thus, chlorophyll a was found to have a similar pattern as N contents under water and N supply. Chlorophyll a was higher in W0N1 than W0N0, and there was no difference in chlorophyll a between W0N0 and W0N2 (Table S1). Increasing chlorophyll contents in W0N1 should lead to the increase in photosynthetic rate (A). However, different from our prediction, one-way ANOVA suggested that A in W0N1 did not differ from that in W0N0 and that A in W0N2 was lower than that in W0N0 (Fig. 2a). Two-way ANOVA showed that N addition had an influence on A (Table 1). Both the analyses suggested that N supply played a negative role in A. These results might be associated with the extremely high light intensity at the study site. Due to the high light intensity, photosynthetic rate might not be correlated with chlorophyll contents (Gabrielsen, 1948). The negative effect of N supply on A led to the decrease in the consumption of intercellular CO2. Consequently, ci ca ratio increased with N supply (Fig. 2d, Table 1). Therefore, the variations in ci ca ratio with N addition could not account for the unchanged pattern in δ13C under N supply (Fig. 1).
The co-application of water and nitrogen had a negative effect on A but no effect on gs (W0N0 vs. W1N1, W1N2, Fig. 2a, b). The responses of A and gs to the co-application of water and nitrogen resulted in an increase in ci ca ratio (Fig. 2d). Since δ13C remained unchanged under the co-application of water and nitrogen (Fig. 1), ci ca ratio could not also explain the observed δ13C response to the co-application of water and nitrogen.
In summary, the unchanged δ13C across treatments was not dependent on the ci ca ratio in H. ammodendron (Fig. 4e). The observed δ13C stability across treatments might be associated with the φ value and carbonic anhydrase (CA) in H. ammodendron. For C4 plants, the relationship between carbon isotope discrimination (Δ≈ δ13Cair−δ13Cplant; see Eq. 2) and ci ca ratio is controlled by φ values (Ellsworth and Cousins, 2016; Ellsworth et al., 2017; Farquhar, 1983; Wang et al., 2008). Some studies suggested that φ value was stable for a given species under a wide range of environmental conditions (Henderson et al., 1992; Wang et al., 2008; Cernusak et al., 2013). However, other studies had different conclusions that φ value was influenced by irradiation (Bellasio and Griffiths, 2014; Kromdijk et al., 2010; Pengelly et al., 2010; Ubierna et al., 2013), temperature (von Caemmerer et al., 2014), water stress (Fravolini et al., 2002; Gong et al., 2017; Williams et al., 2001; Yang et al., 2017) and nitrogen supply (Fravolini et al., 2002; Meinzer and Zhu, 1998; Yang et al., 2017). In the current study, the φ value of H. ammodendron remained unchanged across six treatments (Fig. 5), and two-way ANOVA suggested that water supply and N supply had no effect on φ (Table 1). Therefore, the φ value of H. ammodendron was insensitive to water and N addition in this study. Even if the φ value remains stable, the relationship between Δ and ci ca ratio is also associated with the magnitude of the φ value. Cernusak et al. (2013) predicted that when the φ value is greater than 0.37, the correlation between Δ and ci ca ratio is positive; conversely, when the φ value is less than 0.37, the correlation is negative. In particular, when the φ value is equal to 0.37, there will be no correlation between them, because the coefficient ([] in Eq. 2) of ci ca ratio equals 0 (Cernusak et al., 2013). The φ value calculated from the average δ13Cair ranged from 0.32 to 0.59 with a mean value of 0.45 in the present study. Thus, the correlation between Δ and ci ca in H. ammodendron should be positive based on the prediction by Cernusak et al. (2013). Δ always changes in the opposite direction to δ13Cplant changes according to Eq. (2); thus, a negative relationship between δ13Cplant and ci ca is expected. In fact, this study observed no correlation between δ13C and ci ca in H. ammodendron (Fig. 4e); this indicates that φ was not the driver of the observed δ13C pattern in H. ammodendron. However, the measured δ13C represents the fixed carbon isotope composition throughout the assimilation branch formation period, which usually spans at least several weeks. And the measured ci ca is an instant indicator. As a result, there were some uncertainties in the calculation of φ value using Eq. (2) based on the measured δ13C and ci ca. In addition, the mean φ values calculated from the minimum and maximum δ13Cair were 0.35 and 0.55, respectively, suggesting that the φ value of H. ammodendron might be close to 0.37, which led to the observed insensitive response of δ13C to water and N addition.
The enzymatic activity of CA may be another mechanism behind the unchanged δ13C across treatments. Cousins et al. (2006) suggested that enzymatic activity of CA affects carbon isotope discrimination in most C4 plants because CA can result in the parameter b4 changes (see Eq. 2). But in the traditional view, the parameter b4 was a constant. However, it is only true when the ratio of PEP carboxylation rate to the CO2 hydration rate () is equal to zero, which is caused by a high CA activity. If is not zero, b4 will change and be controlled by (Cousins et al., 2006). Previous studies reported that CA activity was low in most C4 plants (Cousins et al., 2006; Gillon and Yakir, 2000, 2001; Hatch and Burnell, 1990). Thus, CA activity in H. ammodendron might also be low, leading to the change in b4 with , and thus δ13C. Cousins et al. (2006) added into the discrimination pattern of C4 plants and predicted that at a given φ value, when the is 0 or 1, the correlation between Δ and ci ca ratio is negative or positive, respectively. Since CA activity is low in most C4 plants, and the always ranges from 0 to 1, we speculate that no correlation between Δ and ci ca ratio may also occur when the is a certain value between 0 and 1. The irrelevance of Δ and ci ca ratio also means that δ13Cplant is not related to ci ca ratio due to the negative correlation between Δ and δ13Cplant according to Eq. (2). Thus, the uncorrelated pattern between δ13C and ci ca ratio in H. ammodendron might be related to this specific value due to low CA activity.
In addition, the unchanged δ13C across treatments may also be controlled by the water sources of H. ammodendron. A previous study has found that the root of H. ammodendron can be inserted into the soil layer deeper than 3 m (Sheng et al., 2004), which made it easy to uptake groundwater. Therefore, H. ammodendron may be less sensitive to water addition. However, a study conducted in the same region has found that the shallow soil water (0–40 cm) and groundwater are two important water sources for H. ammodendron (Dai et al., 2014), and another study has reported that water addition resulted in an increase in soil water contents in the shallow soil layer (Cui, 2018). Moreover, gas exchange changed across treatments in the present study (Fig. 2). Thus, the utilization of groundwater by H. ammodendron may be one of the reasons why its δ13C was not sensitive to water and N addition, but it should not be the main reason.
Whether foliar δ13C of C4 plants can indicate their WUE is still controversial. Henderson et al. (1992) found that δ13C of 10 C4 species has negative correlation with their WUE. Although this result was just opposite to a positive relationship between δ13C and WUE for C3 plants (Farquhar, 1983; Duquesnay et al., 1998; Feng, 1998), it is proof that δ13C of C4 plants can indicate their WUE. In the work of Henderson et al. (1992), they found that the φ values in 10 C4 species were around 0.21 over a range of irradiance and leaf temperature. According to the suggestion by Cernusak et al. (2013) that Δ is negatively related to ci ca ratio when φ value is less than 0.37, the δ13C of 10 C4 species has a positive correlation with ci ca ratio. In general, under fixed ambient CO2 concentration, WUE is always negatively correlated with ci ca ratio (see Eqs. 4 and 6). This is why a negative relationship between δ13C and WUE was observed for the 10 C4 species. The present study showed that ins-WUE and int-WUE both had no correlation with δ13C in H. ammodendron (Fig. 4a, c), which was different from the results published by Henderson et al. (1992). In general, ci ca ratio is the link between WUE and δ13C. As mentioned above, if the φ value equals 0.37 and/or the activity of CA is very low, δ13C would not correlate to ci ca ratio and thus leads to the uncorrelation between δ13C and WUE. In addition, the different timescales of δ13C, ins-WUE and int-WUE may also result in this uncorrelation. As mentioned above, the measured δ13C represents the long-term fixed carbon isotope composition (at least several weeks). And the values of ins-WUE and int-WUE were calculated from the gas exchange of a short-term measurement, which lasted only a few minutes. Therefore, this difference in timescale may also drive the uncorrelation between δ13C and WUE. Although the defects in measurements could introduce some uncertainty in the observed relationship between δ13C and WUE, δ13C remained stable under water and nitrogen addition (Fig. 1, Table 1), while the measured ins-WUE and int-WUE were higher in the control treatment (W0N0) than other treatments (Fig. 3), suggesting water and N supply had a significant effect on WUE (Table 1). These results indirectly confirmed that δ13C of H. ammodendron could not indicate its WUE.
The present study has found that δ13C of H. ammodendron could not be used as an indicator of its WUE. Although this conclusion cannot be analogous to all C4 plants, the present study has important implications for the understanding of physiological responses of desert plants to future changes in precipitation and atmospheric N deposition. H. ammodendron is a dominant species in Asian desert, which has a great effect on the stabilization of sand dunes, the survival and development of understory plants, and the structure and function of desert ecosystems (Sheng et al., 2005; Su et al., 2007; Cui et al., 2017). Thus, H. ammodendron is widely distributed in desert areas, and the prediction of its drought adaptation is crucial in desert ecosystems.
Global changes including precipitation and atmospheric N deposition have been proven to have an important influence on ecosystems, especially for arid ecosystems. The present study showed that water and N addition had little effect on the δ13C values and the degree of bundle-sheath leakiness (φ) of H. ammodendron but played an important role in the change of its gas exchange and water use efficiency (WUE). In addition, different patterns of instantaneous WUE (ins-WUE), intrinsic WUE (int-WUE) and δ13C across treatment and no correlation between instantaneous WUE (ins-WUE) and δ13C and between intrinsic WUE (int-WUE) and δ13C have been found in this study, suggesting that δ13C of H. ammodendron could not indicate its WUE. This result was caused by the lack of correlation between δ13C and the ratio of intercellular to ambient CO2 concentration (ci ca), which might be associated with the degree of bundle-sheath leakiness (φ) or the low activity of carbonic anhydrase (CA). Thus, the current experiment implies that the availability of δ13C as the indicator of WUE could be not universal for C4 species.
The datasets analyzed in this paper are not publicly available. Requests to access the datasets should be directed to gawang@cau.edu.cn.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-2859-2021-supplement.
GW and JL designed the experiment and modified the manuscript. ZC designed and executed the experiment and wrote the manuscript. XL designed the experiment. XC executed the experiment. YH executed the experiment.
The authors declare that they have no conflict of interest.
This research was supported by the Chinese National Basic Research Program (no. 2014CB954202) and a grant from the National Natural Science Foundation of China (no. 41772171). We are grateful for the support from the Fukang Observation Station of Desert Ecology and Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences. We would like to thank Ma Yan for analyzing stable carbon isotope ratios in the Isotope Lab at the College of Resources and Environment, China Agricultural University.
This research was supported by the Chinese National Basic Research Program (no. 2014CB954202) and a grant from the National Natural Science Foundation of China (no. 41772171).
This paper was edited by Aninda Mazumdar and reviewed by three anonymous referees.
Battipaglia, G., Saurer, M., Cherubini, P., Calfapietra, C., McCarthy, H. R., Norby, R. J., and Cotrufo, M. F.: Elevated CO2 increases tree-level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites, New Phytol., 197, 544–554, 2013.
Bellasio, C., and Griffiths, H.: Acclimation to low light by C4 maize: implications for bundle sheath leakiness, Plant Cell Environ., 37, 1046–1058, 2014.
Cernusak, L. A., Winter, K., Aranda, J., Turner, B. L., and Marshall, J. D.: Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility, J. Exp. Bot., 58, 35490–3566, 2007.
Cernusak, L. A., Ubierna, N., Winter, K., Holtum, J. A. M., Marshall, J.. D., and Farquhar, G. D.: Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants, New Phytol., 200, 950–965, https://doi.org/10.1111/nph.12423, 2013.
Chen, Y., Wang, Q., Li, W., and Ruan, X.: Microbiotic crusts and their interrelations with environmental factors in the Gurbantonggut desert, western China, Environ. Geol., 52, 691–700, 2007.
Cousins, A. B., Badger, M. R., and von Caemmerer, S.: Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis. Insights from antisense RNA in Flaveria bidentis, Plant Physiol., 141, 232–242, 2006.
Craig, H.: Carbon-13 in plants and relationships between carbon-13 and carbon-14 variations in nature, J. Geol., 62, 115–149, 1954.
Cui, X. Q.: Effects of enhanced precipitation, temperature and nitrogen addition on nitrogen fate and plant stoichiometry in temperate desert ecosystem in Xinjiang, PhD thesis, China Agricultural University, Beijing, China, 2018.
Cui, X. Q., Yue, P., Gong, Y., Li, K. H., Tan, D. Y., Goulding, K., and Liu, X. J.: Impacts of water and nitrogen addition on nitrogen recovery in, Haloxylon ammodendron, dominated desert ecosystems, Sci. Total Environ., 601–602, 1280–1288, 2017.
Dai, Y., Zheng, X., Tang, L., and Li, Y.: Dynamics of water usage in Haloxylon ammodendron in the southern edge of the Gurbantunggut Desert, Chinese J. Plant Ecol., 38, 1214–1225, 2014.
Diefendorf, A. F., Mueller, K. E., and Wing, S. L.: Global patterns in leaf 13C discrimination and implications for studies of past and future climate, P. Natl. Acad. Sci. USA, 107, 5738–5743, https://doi.org/10.1073/pnas.0910513107, 2010.
Duquesnay, A., Breda, N., Stievenard, M., and Dupouey, J.: Changes of tree-ring δ13C and water-use efficiency of beech (Fagus sylvatica L.) in northeastern France during the past century, Plant Cell Environ., 21, 565–572, 1998.
Ehleringer, J. R. and Cerling, T. E.: Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants, Tree Physiol., 15, 105–111, 1995.
Ellsworth, P. Z. and Cousins, A. B.: Carbon isotopes and water use efficiency in C4 plants, Curr. Opin. Plant. Biol., 31, 155–161, 2016.
Ellsworth, P. Z., Ellsworth, P. V., and Cousins, A. B.: Relationship of leaf oxygen and carbon isotopic composition with transpiration efficiency in the C4 grasses Setaria viridis and Setaria italica, J. Exp. Bot., 68, 3513–3528, 2017.
Fan, L. L., Li, Y., Tang, L. S., and Ma, J.: Combined effects of snow depth and nitrogen addition on ephemeral growth at the southern edge of the Gurbantunggut Desert, China, J. Arid. Land, 5, 500–510, 2013.
Farquhar, G. D.: On the nature of carbon isotope discrimination in C4 species, Aust. J. Plant Physiol., 10, 205–226, 1983.
Farquhar, G. D. and Richards, P. A.: Isotopic composition of plant carbon correlates with water-use efficiency of wheat gemotypes, Aust. J. Plant Physiol., 11, 539–552, 1984.
Feng, X.: Long-term response of trees in western North America to atmospheric CO2 concentration derived from carbon isotope chronologies, Oecologia, 117, 19–25, 1998.
Frank, D., Reichstein, M., Bahn, M., Thonicke, K., Frank, D., Mahecha, M. D., Smith, P., der Velde, M. V., Vicca, S., Babst, F., Beer, C., Buchmann, N, Canadell, J. C., Ciais, P., Cramar, W., SIbrom, A., Miglietta, F., Poulter, B., Rammig, A., Seneviratne, S. I., Walz, A., Wattenbach, M., Zavala, M. A., and Zscheischler, J.: Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts, Glob. Change Biol., 21, 2861–2880, 2015.
Fravolini, A., Williams, D. G., and Thompson, T. L.: Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply, J. Exp. Bot., 53, 2261–2269, 2002.
Gabrielsen, E. K.: Effects of different chlorophyll concentrations on photosynthesis in foliage leaves, Physiol. Plantarum, 1, 5–37, 1948.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., Asner, G. P., Cleveland, C. C., Green, P. A., Holland, E. A., Karl, D. M., Michaels, A. F., Porter, J. H., Townsend, A. R., and Vörösmarty, C. J.: Nitrogen cycles: past, present, and future, Biogeochemistry, 70, 153–226, 2004.
Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., Martinelli, L. A., Seitzinger, S. P., and Sutton, M. A.: Transformation of the nitrogen cycle: recent trends, questions, and potential solutions, Science, 320, 889–892, 2008.
Gillon, J. S. and Yakir, D.: Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against (COO)-O-18 during photosynthesis, Plant Cell Environ., 23, 903–915, 2000.
Gillon, J. S. and Yakir, D.: Influence of carbonic anhydrase activity in terrestrial vegetation on the O-18 content of atmospheric CO2, Science, 291, 2584–2587, 2001.
Gong, X. Y., Schäufele, R., and Schnyder, H.: Bundle-sheath leakiness and intrinsic water use efficiency of a perennial C4 grass are increased at high vapour pressure deficit during growth. J. Exp. Bot., 68, 321–333, 2017.
Gong, X. W, Lü, G. H., He, X. M., Sarkar, B., and Yang X. D.: High air humidity causes atmospheric water absorption via assimilating branches in the deep-rooted tree Haloxylon ammodendron in an arid desert region of Northwest China, Front. Plant Sci., 10, 573, https://doi.org/10.3389/fpls.2019.00573, 2019.
Gresset, S., Westermeier, P., Rademacher, S., Ouzunova, M., Presterl, T., Westhoff, P., and Schön, C.: Stable carbon isotope discrimination is under genetic control in the C4 species maize with several genomic regions influencing trait expression, Plant Physiol., 164, 131–143, 2014.
Hall, S. J., Sponseller, R. A., Grimm, N. B., Huber, D., Kaye, J. P., Clark, C., and Collins, S. L.: Ecosystem response to nutrient enrichment across an urban airshed in the Sonoran Desert, Ecol. Appl., 21, 640–660, 2011.
Hatch, M. D. and Burnell, J. N.: Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis, Plant Physiol., 93, 825–828, 1990.
Henderson, S. A., von Caemmerer, S., and Farquhar, G. D.: Short-termmeasurements of carbon isotope discrimination in several C4 species, Aust. J. Plant Physiol., 19, 263–285, 1992.
Huang, J. P., Yu, H. P., Guan, X. D., Wang, G. Y., and Guo, R. X.: Accelerated dryland expansion under climate change, J. Nature Climat. Chang., 6, 166–171, https://doi.org/10.1038/NCLIMATE2837, 2016.
Huang, J. Y., Wang, P., Niu, Y. B., Yu, H. L., Ma, F., Xiao, G. J., and Xu, X.: Changes in C : N : P stoichiometry modify N and P conservation strategies of a desert steppe species Glycyrrhiza uralensis, Sci. Rep.-UK, 8, 12668, https://doi.org/10.1038/s41598-018-30324-w, 2018.
Knapp, A. K., Hoover, D. L., Wilcox, K. R., Avolio, M. L., Koerner, S. E., La Pierre, K. J., Loik, M. E., Luo Y. Q., Sala, O. E., and Smith, M. D.: Characterizing differences in precipitation regimes of extreme wet and dry years: Implications for climate change experiments, Glob. Change Biol., 21, 2624–2633, 2015.
Kohn, M. J.: Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate, P. Natl. Acad. Sci. USA, 107, 19691–19695, https://doi.org/10.1073/pnas.1004933107, 2010.
Kromdijk, J., Griffiths, H., and Schepers, H. E.:. Can the progressive increase of C4 bundle sheath leakiness at low PFD be explained by incomplete suppression of photorespiration?, Plant Cell Environ., 33, 1935–1948, 2010.
Li, J. Z., Wang, G. A., Zhang, R. N., and Li, L.: A negative relationship between foliar carbon isotope composition and mass-based nitrogen concentration on the eastern slope of mount gongga, China, PLoS ONE, 11, e0166958, https://doi.org/10.1371/journal.pone.0166958, 2016.
Liu, W. G., Feng, X. H., Ning, Y. F., Zhang, Q. L., Cao, Y. N., and An, Z. S.: δ13C variation of C3 and C4 plants across an asian monsoon rainfall gradient in arid northwestern China, Glob. Change Biol., 11, 1094–1100, 2005.
Liu, X., Zhang, Y., Han, W., Tang, A., Shen, J., Cui, Z., Vitousek, P., Erisman, J. W., Goulding, K., Christie, P., Fangmeier, A., and Zhang, F.: Enhanced nitrogen deposition over China, Nature, 494, 459–462, 2013.
Liu, Y. X., Li, X., Zhang, Q., Guo, Y. F., Gao, G., and Wang, J. P.: Simulation of regional temperature and precipitation in the past 50 years and the next 30 years over China, Quatern. Int., 212, 57–63, 2010.
Ma, J. Y., Sun, W., Liu, X. N., and Chen, F. H.: Variation in the stable carbon and nitrogen isotope composition of plants and soil along a precipitation gradient in northern China, PLoS ONE, 7, e51894, https://doi.org/10.1371/journal.pone.0051894, 2012.
Ma, J. Y., Sun, W., Koteyeva, N. K., Voznesenskaya, E., Stutz, S. S., Gandin, A., Smith-Moritz, A. M., Heazlewood, J. L., and Cousins, A. B.: Influence of light and nitrogen on the photosynthetic efficiency in the c4 plant Miscanthus × Giganteus, Photo. Res., 131, 1–11, 2016.
Meinzer, F. C. and Zhu, J.: Nitrogen stress reduces the efficiency of the C4 CO2 concentrating system, and therefore quantum yield, in Saccharum (sugarcane) species, J. Exp. Bot., 49, 1227–1234, 1998.
Nyongesah, M. J. and Wang, Q.: Variation of photosynthesis and pigment concentration relative to irradiance and nitrogen content for two coexisting desert shrubs. Ecol. Eng., 58, 238–248, 2013.
O'Leary, M. H.: Measurement of the isotopic fractionation associated with diffusion of carbon dioxide in aqueous solution, J. Phys. Chem., 88, 823–825, 1984.
Pengelly, J. J. L., Sirault, X. R. R., Tazoe, Y., Evans, J. R., Furbank, R. T., and von Caemmerer, S.: Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry, J. Exp. Bot., 61, 4109–4122, 2010.
Rao, Z. G., Guo, W. K., Cao, J. T., Shi, F. X., Jiang, H., and Li, C. Z.: Relationship between the stable carbon isotopic composition of modern plants and surface soils and climate: A global review, Earth Sci. Rev., 165, 110–119, 2017.
Reynolds, J. F., Smith, D. M. S., Lambin, E. F., Turnerll, B. L., Mortimore, M., Batterbury, S. P. J., Downing, T. E., Dowlatabadi, H., Fernández, R. J., Herrick, J. E., Huber-Sannwald, E., Jiang, H., Leemans, R., Lynam, T., Maestre, F. T., Ayarza, M., and Walker, B.: Global desertification: building a science for dryland development, Science, 316, 847–851, 2007.
Schmidt, G., Gebauer, G., Widmann, K., and Ziegler, H.: Influence of nitrogen supply and temperature on stable carbon isotope ratios in plants of different photosynthetic pathways (C3, C4, CAM), Isot. Environ. Healt. S., 29, 9–13, 1993.
Serret, M. D., Yousfi, S., Vicente, R., Piñero, M. C., Otálora-Alcón G., del Amor, F. M., and Araus, J. L.: Interactive effects of CO2 concentration and water regime on stable isotope signatures, nitrogen assimilation and growth in sweet pepper, Front. Plant Sci., 8, 2180, https://doi.org/10.3389/fpls.2017.02180, 2018.
Sheng, J., Qiao, Y., Liu, H., Zhai, Z., and Guo, Y.: A Study on the Root System of Haloxylon Aammodendron (C. A. Mey.) Bunge, Acta Agrestia Sinica, 12, 91–94, 2004.
Sheng, Y., Zheng, W., Pei, K., and Ma, K.: Genetic variation within and among populations of a dominant desert tree Haloxylon ammodendron (Amaranthaceae) in China, Ann. Bot., 96, 245–252, 2005.
Song, L., Kuang, F., Skiba, U., Zhu, B., Liu, X., Levy, P., Dore, A., and Fowler, D.: Bulk deposition of organic and inorganic nitrogen in southwest China from 2008 to 2013, Environ. Pollut., 227, 157–166, 2017.
Sparks, J. P. and Ehleringer, J. R.: Leaf carbon isotope discrimination and nitrogen content for riparian trees along elevational transects, Oecologia, 109, 362–367, https://doi.org/10.1007/s004420050094, 1997.
Stewart, G. R., Turnbull, M. H., Schmidt, S., and Erskine, P. F.: 13C Natural abundance in plant communities along a rainfall gradient: a biological integrator of water availability, Aust. J. Plant Physiol., 22, 51–55, https://doi.org/10.1071/PP9950051, 1995.
Swap, R. J., Aranibar, J. N., Dowty, P. R., Gilhooly III, W. P., and Macko, S. A.: Natural abundance of 13C and 15N in C3 and C4 vegetation of southern africa: patterns and implications. Glob. Change Biol., 10, 350–358, 2004.
Tranan, M. W. and Schubertt, B. A.: Temperature-induced water stress in high-latitude forests in response to natural and anthropogenic warming, Glob. Change Biol., 22, 782–791, https://doi.org/10.1111/gcb.13121, 2016.
Ubierna, N., Sun, W., Kramer, D. M., and Cousins, A. B.: The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus x giganteus and Flaveria bidentis, Plant Cell Environ., 36, 365–381, 2013.
von Caemmerer, S., Ghannoum, O., Pengelly, J. J. L., and Cousins, A. B.: Carbon isotope discrimination as a tool to explore C4 photosynthesis, J. Exp. Bot., 65, 3459–3470, 2014.
Wang, G., Feng, X., Han, J., Zhou, L., Tan, W., and Su, F.: Paleovegetation reconstruction using δ13C of Soil Organic Matter, Biogeosciences, 5, 1325–1337, https://doi.org/10.5194/bg-5-1325-2008, 2008.
Wang, G. A. and Feng, X. H.: Response of plants' water use efficiency to increasing atmospheric CO2 concentration, Environ. Sci. Technol., 46, 8610–8620, 2012.
Wang, G. A., Han, J. M., Zhou, L. P., Xiong, X. G., and Wu, Z. H.: Carbon isotope ratios of plants and occurrences of C4 species under different soil moisture regimes in arid region of Northwest China, Physiol. Plant., 25, 74–81, 2005.
Wang, G. A., Han, J. M., Zhou, L. P., Xiong, X. G., Tan, M., Wu, Z. H., and Peng, J.: Carbon isotope ratios of C4 plants in loess areas of North China, Sci. China Ser. D., 49, 97–102, 2006.
Williams, D. G., Gempko, V., Fravolini, A., Leavitt, S. W., Wall, G. W., Kimball, B. A., Pinter Jr., P. J., LaMorte, R., and Ottman, M.: Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought, New Phytol., 150, 285–293, 2001.
Yang, H., Yu, Q., Sheng, W. P., Li, S. G., and Tian, J.: Determination of leaf carbon isotope discrimination in C4 plants under variable N and water supply, Sci. Rep.-UK, 7, 351, https://doi.org/10.1038/s41598-017-00498-w, 2017.
Yao, F. Y., Wang, G. A., Liu, X. J., and Song, L.: Assessment of effects of the rising atmospheric nitrogen deposition on nitrogen uptake and long-term water-use efficiency of plants using nitrogen and carbon stable isotopes, Rapid Commun. Mass Sp., 25, 1827–1836, 2011.
Zhang, J., Gu, L., Bao, F., Cao, Y., Hao, Y., He, J., Li, J., Li, Y., Ren, Y., Wang, F., Wu, R., Yao, B., Zhao, Y., Lin, G., Wu, B., Lu, Q., and Meng, P.: Nitrogen control of 13C enrichment in heterotrophic organs relative to leaves in a landscape-building desert plant species, Biogeosciences, 12, 15–27, https://doi.org/10.5194/bg-12-15-2015, 2015.
Zhang, Y. M., Chen, J., Wang, L., Wang, X. Q., and Gu, Z. H.: The spatial distribution patterns of biological soil crusts in the Gurbantunggut Desert, Northern Xinjiang, China, J. Arid Environ., 68, 599–610, 2007.