the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Carbon sources of benthic fauna in temperate lakes across multiple trophic states
Eva Anthamatten
Longhui Deng
Xingguo Han
Lorenzo Lagostina
Anja Michel
Rong Zhu
Nathalie Dubois
Carsten J. Schubert
Stefano M. Bernasconi
Mark A. Lever
Previous studies have shown that microbially produced methane can be a dominant carbon source of lacustrine sedimentary macrofauna in eutrophic lakes, most likely through grazing on methane-oxidizing bacteria. Here we investigate the contributions of different carbon sources to macrofaunal biomass across five lakes in central Switzerland that range from oligotrophic to highly eutrophic. Macrofaunal communities change with trophic state, with chironomid larvae dominating oligotrophic and tubificid oligochaetes dominating eutrophic lake sediments. The 13C-isotopic data suggest that the average contribution of methane-derived carbon to the biomass of both macrofaunal groups is similar but consistently remains minor, ranging from only ∼1 % in the oligotrophic lake to at most 12 % in the eutrophic lakes. The remaining biomass can be explained by the assimilation of detritus-derived organic carbon. Low abundances of methane-cycling microorganisms in macrofaunal specimens, burrows, and surrounding sediment based on 16S ribosomal RNA (rRNA) gene sequences and copy numbers of genes involved in anaerobic and aerobic methane cycling (mcrA, pmoA) support the interpretation of isotopic data. Notably, 16S rRNA gene sequences of macrofauna, including macrofaunal guts, are highly divergent from those in tubes or sediments. Many macrofaunal specimens are dominated by a single 16S rRNA phylotype of Fusobacteria, α-, β-, γ-, or ε-Proteobacteria, Bacteroidetes, or Parcubacteria. This raises the question of whether dominant lake macrofauna live in so far uncharacterized relationships with detrital organic-matter-degrading bacterial endosymbionts.
- Article
(1929 KB) - Full-text XML
-
Supplement
(3026 KB) - BibTeX
- EndNote
Lake sediments are globally important organic C sinks (Einsele et al., 2001; Mendonça et al., 2017) and sources of the greenhouse gas methane (CH4) (Bastviken et al., 2004; Raymond et al., 2013; Holgerson and Raymond, 2016). Overall the burial of organic carbon is usually higher in eutrophic compared to oligotrophic lakes due to high nutrient loads which increase primary production (Dean and Gorham, 1998; Maerki et al., 2009; Heathcote and Downing, 2012; Anderson et al., 2013; Anderson et al., 2014). The resulting increases in aerobic respiration lead to O2 depletion and increased organic matter deposition to sediments (Hollander et al., 1992; Steinsberger et al., 2017) where this increased organic matter stimulates microbial methane production (Fiskal et al., 2019). The combination of increased methane production in sediments and decreased aerobic methane consumption in overlying water then results in higher methane emissions from eutrophic lakes (DelSontro et al., 2016).
In addition to trophic state, the presence of macrofauna, which physically mix sediments, mechanically break down organic particles, or pump O2 into deeper, otherwise anoxic layers, influences O2 and C cycle dynamics in sediments (Meysman et al., 2006; White and Miller, 2008; Kristensen et al., 2012). While most research on macrofaunal effects on organic carbon burial and respiration reactions have been on marine sediments, there have also been numerous studies on freshwater sediments. These studies suggest that macrofauna can be present in high abundances (up to 11 000 individuals per square meter) (Armitage et al., 1995; Mousavi, 2002) and significantly influence nutrient fluxes and sedimentary matrices in lake sediments (Stief, 2013; Holker et al., 2015). Insects, in particular tube-dwelling chironomid larvae, can cause oxic–anoxic oscillations around their burrows through their pumping activity (Lewandowski et al., 2007; Roskosch et al., 2012; Baranov et al., 2016; Hupfer et al., 2019) These redox fluctuations affect the sedimentary cycles of nitrogen (Pelegri et al., 1994; Jeppesen et al., 1998; Stief et al., 2009; Stief, 2013), phosphorus (Andersson et al., 1988; Katsev et al., 2006), iron (Hupfer and Lewandowski, 2008), and methane (Deines et al., 2007b; Gentzel et al., 2012). Worms, especially tubificid oligochaetes, can also increase oxygenation and O2 uptake in surface sediments (Lagauzère et al., 2009) and influence the release of ammonium (), nitrate (), and phosphate () (Svensson et al., 2001; Mermillod-Blondin et al., 2005; Gautreau et al., 2020) from surface sediments. Many tubificids are moreover head-down deposit feeders that defecate on the sediment surface (McCall and Tevesz, 1982). This upward movement of reduced sediment can cause significant reworking and alter the redox potential in surface sediment (Davis, 1974).
The community composition of lacustrine sedimentary macrofauna varies in response to trophic state (Aston, 1973; Verdonschot, 1992; Nicacio and Juen, 2015) in part due to differences in hypoxia/anoxia tolerance among macrofaunal species (Chapman et al., 1982). Different lacustrine macrofaunal species, moreover, vary in their impact on methane cycling in sediments (Bussmann, 2005; Figueiredo-Barros et al., 2009). methane oxidation in surface sediments is often stimulated by chironomid larval O2 input, which enriches populations of methane-oxidizing bacteria in larval tubes and surrounding sediment (“microbial gardening”) (Kajan and Frenzel, 1999). As a result, methane-oxidizing bacteria can become an important food source, and in some cases the main C source, of chironomid larvae (Kankaala et al., 2006; Deines et al., 2007a; Jones et al., 2008; Jones and Grey, 2011). High contributions of methane-derived carbon via grazing on methane-oxidizing bacteria are typically found in profundal regions of eutrophic lakes with seasonal stratification and low O2 concentrations (Hershey et al., 2006; Jones and Grey, 2011). Yet, variable isotopic values of chironomid biomass, even within the same location, suggest that diets of chironomid larvae vary greatly (Kiyashko et al., 2001; Reuss et al., 2013). The limited C-isotopic data on tubificid worms suggest that worm C sources also vary from detritus-based to locally or seasonally high contributions of methane-derived carbon (Premke et al., 2010).
Despite these past studies, the conditions under which methane-derived carbon becomes an important C source to chironomid larvae or oligochaetes are not well understood. Furthermore, the main pathways of methane-derived carbon incorporation into macrofaunal biomass, e.g., selective grazing or gardening of methane-oxidizing bacteria or carbon transfer from methane-oxidizing bacteria gut symbionts, remain unclear. Here we analyze shallow sublittoral to profundal sediments of five temperate lakes in central Switzerland that differ strongly in trophic state and macrofaunal community composition. We analyze the community structures of chironomid larvae and oligochaetes and compare their C-isotopic compositions to those of total organic C (TOC), dissolved organic C (DOC), and methane to investigate how C sources vary across dominant macrofaunal groups in relation to trophic state and water depth. In addition, we analyze microbial community structure based on 16S rRNA gene sequences and quantify functional genes involved in aerobic and anaerobic methane oxidation in macrofaunal specimens, macrofaunal burrows, and surrounding sediment to elucidate the potential for microbial gardening or symbiotic associations between macrofauna and microbiota.
Figure 1Map of the study area from Fiskal et al., (2019). The sampling stations within each of the five lakes are indicated by red dots and numbered 1 to 3. Color indicates trophic state from light blue (oligotrophic) to dark blue (eutrophic). The map is based on aerial images from DigitalGlobe (CO) and CNES/Airbus (France) as provided by Google (CA) and was created with the software R (South, 2011). The small insert map is from d-maps (https://www.d-maps.com/carte.php?num_car=2648&lang=en).
Table 1Overview of sampled lakes, their trophic status, and maximum water depths, as well as the geographic coordinates, water depths, and bottom water dissolved O2 concentrations (ranges are O2 concentrations over the time course of 1 year) of the stations that were sampled. O2 concentrations ≤15.6 µM are termed “hypoxic”. All data are from Fiskal et al., (2019). Trophic status and O2 concentrations are taken from Swiss Federal Office of the Environment (BAFU) (https://www.bafu.admin.ch/bafu/de/home/themen/wasser/fachinformationen/zustand-der-gewaesser/zustand-der-seen/wasserqualitaet-der-seen.html).
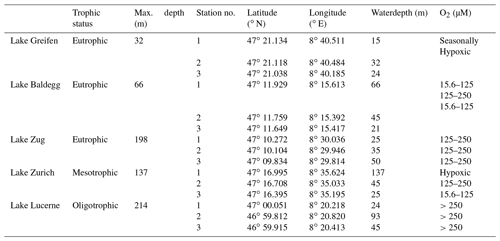
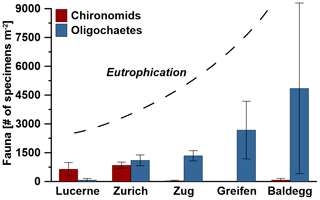
Figure 2Average abundances of macrofauna in each lake. Error bars indicate standard deviations of three stations per lake, except for Lake Zurich where the macrofauna-free deep station was not considered, and error bars indicate the range of the two shallower stations. The degree of eutrophication is based on water column phosphorous concentrations measured by the Swiss Federal Office of the Environment (BAFU), which uses the OECD model (Vollenweider and Kerekes, 1982) to declare trophic state. According to the OECD model, lakes with average total P concentration values of ≤15 mg m−3 are oligotrophic, lakes with 15–45 mg P m−3 are mesotrophic, and lakes with >45 mg P m−3 are eutrophic.
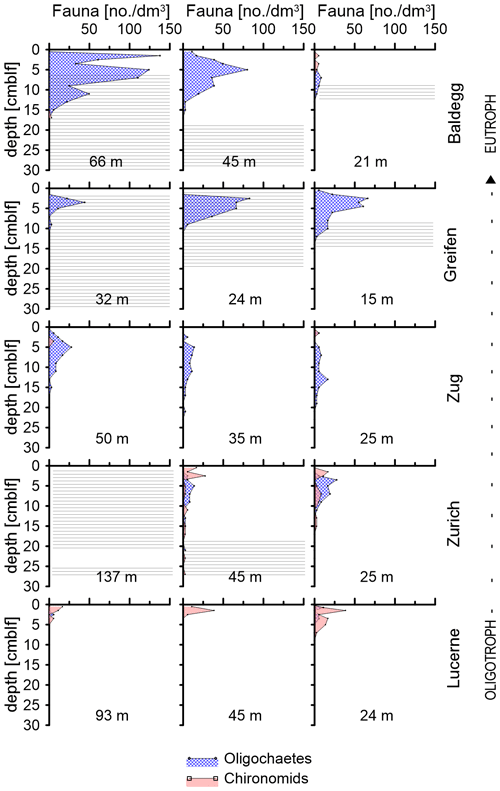
Figure 3Depth distributions of oligochaetes and chironomid larvae at each station. Water depths of each station are indicated in each subplot. Horizontal lines indicate depth distributions of laminated sediment layers.
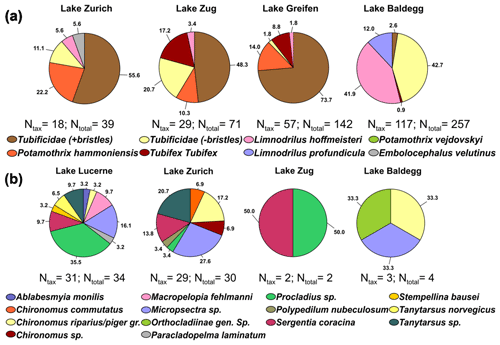
Figure 4Pie charts of taxonomic analyses on oligochaetes (a) and chironomid larvae (b) in each lake (Ntax= number of taxonomically identified specimens, Ntotal= total number of specimens). No chironomid larvae were found in Lake Greifen. In Lake Lucerne only four oligochaetes were found of which one was taxonomically analyzed (Potamothrix vejdovskyi; not shown). Numbers show relative abundances in percentages.
2.1 Sampling and site description
Sediment cores were obtained from three different water depths in the oligotrophic Lake Lucerne, the mesotrophic Lake Zurich, and the eutrophic Lake Zug, Lake Baldegg, and Lake Greifen in central Switzerland in June and July 2016 (Fig. 1, Table 1; for further information on trophic histories, please see Fiskal et al., 2019). Sediment cores were taken using gravity cores with 60 cm long liners that had an inner diameter of 150 mm (UWITEC, AT) from boats or motorized platforms. The four sediment cores per station were used as follows: the most undisturbed core was used for microsensor measurements (O2, pH) and afterwards for macrofaunal community sampling. The second core was used for analyses of DNA sequences, methane concentrations, δ13C-methane, TOC content, and δ13C-TOC. The remaining cores were used for porewater sampling using rhizons (0.2 µm pore size, Rhizosphere), with DOC and δ13C-DOC sampling being done on a separate core than that for dissolved inorganic carbon (DIC) and δ13C-DIC sampling. A wide range of additional porewater geochemical analyses were performed on the core used for DIC sampling (including concentrations of nitrate, sulfate, hydrogen sulfide, Fe2+, Mn2+, and ammonium; for further details, see Fiskal et al., 2019). In all cores, the top 4 cm was sampled in 0.5 to 1 cm depth intervals, samples from 4 to 20 cm sediment depth in 2 cm depth intervals, and all deeper layers in 4 cm depth intervals. Cores were typically ∼40–50 cm long; however, the lowermost 5–10 cm was discarded due to contamination with lake water during core retrieval. An additional, narrow core 6 cm in diameter was obtained for radionuclide (210Pb and 137Cs) analyses (for analytical details, see Fiskal et al., 2019). Cores for macrofaunal community analyses were extruded and macrofauna collected by sieving sediments through 400 and 200 µm mesh sieves. Three stations (two in Lake Lucerne, one in Lake Baldegg) were revisited in November 2017 and October 2018 to collect additional macrofaunal specimens and chironomid larval tubes for DNA analyses.
2.2 Macrofaunal abundance and taxonomy
For each depth interval, specimen numbers of oligochaetes and chironomid larvae were carefully picked with tweezers, counted, and preserved in 70 % ethanol for taxonomic and 13C-isotopic analyses or frozen on dry ice for DNA extractions. Detailed taxonomic analyses to the genus and, when possible, species level were performed on a subset of oligochaetes and chironomid larvae. Oligochaete specimens were sent to AquaLytis (Wildau, Germany), where they were embedded in epoxy resin and identified by light microscopy. Chironomid larvae were microscopically identified by AquaDiptera (Emmendingen, Germany).
2.3 Stable carbon isotope analyses
Carbon isotope analyses were performed on DOC, methane, TOC, macrofaunal specimens, or separately on guts and remaining bodies of macrofaunal specimens. Values are given in the δ notation, i.e.,
For δ13C-DOC, porewater samples were analyzed as described in Lang et al. (2012). Briefly, 2–7 mL of sample was added to 12 mL Vacutainers®. After removal of dissolved inorganic C by addition of 85 % phosphoric acid and bubbling with high purity He, DOC was oxidized to CO2 using persulfate (1 h at 100∘C). The evolved CO2 was analyzed on a GasBench II coupled to a Delta V mass spectrometer (Thermo Fisher Scientific, Bremen). Water soluble organic standards of known isotope composition (phthalic acid and sucrose) were used as standards.
For δ13C-Methane, methane was extracted by creating a sediment slurry with Milli-Q water under saturating NaCl concentrations (∼6.3 M). A total of 2 cm3 of sediment was transferred to 20 mL crimp vials containing 2.514 g NaCl and 5 mL Milli-Q water, crimped, mixed, and stored on ice or at +4 ∘C until analysis using a trace gas (Isoprime) coupled to an isotope ratio mass spectrometer (GC-IRMS; Isoprime, Manchester). Separation was performed through a GC-column (PoraPLOT Q 30 m column). The precision of the method was ±0.7 %. After every sixth sample we included a standard with a known δ13C value (standards: L-iso1 with 2500 ppmv CH4 at −66.5 % δ13C-methane and T-iso3 with 250 ppmv CH4 at −38.3 % δ13C-CH4; Air Liquide).
For δ13C-TOC analyses, 5–10 g of frozen sediment was freeze-dried in glass vials and quantified using an elemental analyzer (Thermo Fisher Flash EA 1112) coupled to an isotope-ratio mass spectrometer (Thermo Fisher Delta V Plus) (EA-IRMS) as outlined in Fiskal et al. (2019).
For δ13C-Macrofauna, δ13C-analyses were performed on macrofaunal biomass according to the same method used for δ13C-TOC. Single specimens were cleaned with molecular grade water to remove sediment. Whole organisms, or separated guts and residual bodies, were placed in tin foil capsules, which were mounted to 96-well plates. The 96-well plates were sealed using plastic seal foil, the foil above each well was pierced, and the whole plate was freeze dried. Afterward, the foil was removed, the tin foil capsules were closed, and the δ13C of macrofaunal biomass was measured.
2.4 Two end-member mixing model
Assuming TOC and methane as the only carbon sources, a two end-member mixing model was used to estimate the contribution of methane to biomass C of macrofauna.
2.5 DNA extraction from macrofauna, macrofaunal tubes, and sediment
DNA was extracted according to lysis protocol II of the modular DNA extraction method of Lever et al. (2015) following the exact procedure outlined in Han et al. (2020). While we used existing sediment DNA extracts from the latter study, DNA from empty larval tubes and from macrofauna were newly extracted. To remove sediment, macrofaunal specimens were rinsed with molecular grade water. DNA was then extracted from entire specimens, or separately on guts and the remaining body, after being cut into three to four pieces to increase extraction efficiency using a sterile scalpel. All DNA extracts were stored at −80 ∘C.
2.6 Quantitative polymerase chain reaction
Quantitative polymerase chain reaction (qPCR) was performed to quantify bacterial and archaeal 16S rRNA genes, as well as genes encoding particulate methane monooxygenase of aerobic methanotrophic bacteria (pmoA) and methyl coenzyme M reductase of methanogenic and anaerobic methane-oxidizing archaea (mcrA) (Table S1 in the Supplement). Standards consisting of plasmids containing 16S rRNA, pmoA, or mcrA genes from specific organisms (Table S1 in the Supplement) were run in 10-fold dilutions of ∼101 to ∼107 gene copies per qPCR reaction. All sample DNA extracts and standard dilutions were run in duplicate.
The qPCR protocols are shown in Table S2 in the Supplement. For each qPCR reaction, 2 µL of DNA extract was mixed with 1 µL of molecular grade water, 1 µL of bovine serum albumin (10 mg mL−1; New England Biolabs, USA), 0.5 µL each of forward and reverse primers (10 µM), and 5 µL LightCycler® 480 SYBR Green I Master Mix (Roche, Switzerland). All standards and samples were kept on ice throughout the preparations and run immediately after in transparent 96-well plates on a Roche LightCycler® 480.
2.7 Next generation sequencing (NGS) and bioinformatics analyses
Libraries of bacterial and archaeal communities were produced using the universal 16S rRNA primer pair Univ519F and Univ802R (Claesson et al., 2009; Wang and Qian, 2009). After library preparation DNA was pooled and sequenced using a MiSeq (Illumina Inc., USA). Library preparations and subsequent data processing, including 97 % zero-radius operational taxonomic unit (ZOTU) clustering, were done as outlined in Han et al. (2020) (for polymerase chain reaction mixtures and cycler conditions, see Table S3 in the Supplement). Briefly, raw sequences were initially quality trimmed using seqtk (https://github.com/lh3/seqtk) and paired-end reads were merged using flash (Magoč and Salzberg, 2011). This was followed by a final quality filtering using prinseq (Schmieder and Edwards, 2011). Sequences were then used to generate ZOTUs with USEARCH unoise3 using a 97 % clustering identity (Edgar, 2016).
2.8 Statistical analyses
Statistical differences between C isotope signatures of macrofauna and C pools, as well as of percentages of bacterial 16S rRNA, mcrA, and pmoA gene copy numbers relative to total 16S rRNA gene copy numbers across macrofauna, larval tubes, and sediment, were determined using Wilcoxon signed rank tests for paired data. All tests were performed in R (Team, 2018) using the following command: wilcox.test (A, B, paired = TRUE, alternative = “two.sided” for (a), “greater/less” for (b), mu = 0.0, exact = TRUE, correct = TRUE, conf.int = TRUE, conf.level = 0.95). Principal coordinates analysis (PCoA) on bacterial communities at the phylum, class, order, family, and genus levels was performed using Bray–Curtis distances in R (Team, 2018).
3.1 Macrofaunal distribution in relation to lake trophic state
Macrofauna are present at all stations except the hypoxic deep station of Lake Zurich and are dominated by oligochaetes and chironomid larvae. While oligochaetes are present in all lakes, no chironomid larvae were found in Lake Greifen. Oligochaete densities increase with trophic state, from 75±86 individuals per square meter in Lake Lucerne to 4849±4443 individuals per square meter in Lake Baldegg (number of individuals are expressed as averages per lake with standard deviations of three stations). Numbers of chironomid larvae show the opposite trend, decreasing from 641±346 individuals per square meter in Lake Lucerne and 849±160 individuals per square meter in Lake Zurich to less than 75±86 individuals per square meter in the three eutrophic lakes (Fig. 2, Table S5 in the Supplement). Other macrofauna, e.g., copepods, Daphnia, and leeches, were only occasionally found and will not be discussed further.
The depth distributions of oligochaetes and chironomid larvae follow different trends (Fig. 3). Chironomid larvae are most abundant in surface sediment (0–5 cmblf, centimeters below lake floor), while oligochaetes occur over a greater depth interval (Fig. 3). In Lake Greifen and Lake Baldegg, oligochaetes are present in high numbers to 12 and 15 cm sediment depth, respectively, including layers that are distinctly laminated (see horizontal lines in Fig. 3). In Lake Zug, oligochaetes are present to even greater depths (22 cm). In sediments of Lake Zurich, where oligochaetes and chironomids occur in similar abundances, chironomids dominate the top ∼2–3 cm, whereas oligochaetes dominate below. Despite depth ranges extending significantly below the sediment surface, macrofaunal sediment reworking is minimal based on radionuclide measurements. These show 137Cs peaks that match the 1986 (Chernobyl) and 1963 (bomb test) time markers, and clear decreases from the top 2 cm downward at all faunated stations (Fig. S6 in the Supplement; data analyzed but not shown in Fiskal et al., 2019). Light microscopic images of the two dominant macrofaunal groups and depth distributions of individual macrofaunal species can be found in Fig. S1 in the Supplement.
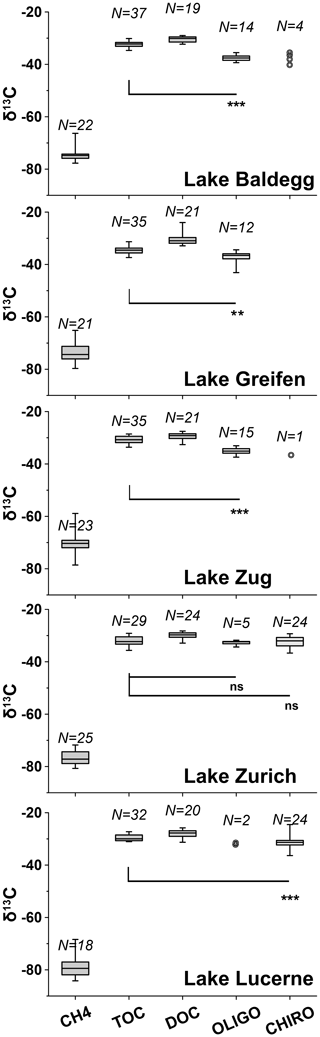
Figure 5Boxplots of 13C isotopic compositions of CH4, TOC, DOC, oligochaetes, and chironomid larvae for each lake (note: no larvae were found in Lake Greifen). Boxes show 75 % and 25 % quartiles. Whiskers show minimum and maximum values. Wilcoxon signed rank tests were applied to check whether 13C-isotopic signatures of macrofauna and TOC were significantly different (ns = not significant; * = p<0.05; ** = p<0.01; *** = p<0.001). For each Wilcoxon test, macrofaunal specimens were paired with TOC isotopic signatures from the same depth (±2 cm), and only data were included for which there were data macrofauna and TOC data from matching depths. Samples with N<5 are displayed as individual data points. N indicates the number of data points for each variable above.
Table 2Contributions of TOC and methane to oligochaete and chironomid larval biomass C based on a two end-member mixing model. Estimates outside of the parentheses are maximum values as they assume no isotopic fractionation during aerobic methane oxidation. Values within parentheses are more conservative and assume a fractionation factor that is in the upper range previously determined for freshwater sediments and pure-culture incubations (−39 %) (Kruger et al., 2002; Templeton et al., 2006; Kankaala et al., 2007). For the calculations, only macrofaunal specimens were included that could be paired with TOC and methane isotopic values from the same sediment depth (±2 cm); values display averages ± standard deviation.

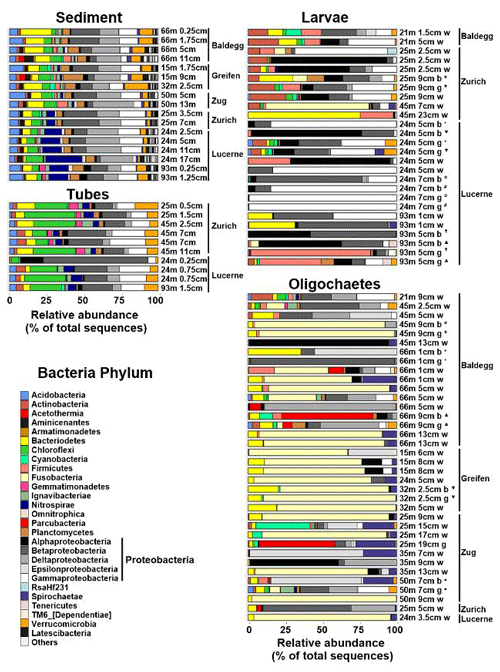
Figure 6Relative abundances of Bacteria at the phylum level (Proteobacteria at class level) based on 16S rRNA gene sequences. Sequences were obtained from 17 sediment, 10 chironomid larval tube, 26 chironomid larvae (Nbody= 7, Ngut= 7, Nwhole= 12), and 36 oligochaete (Nbody= 5, Ngut= 6, Nwhole= 25) samples. Station and sample IDs are indicated by sample names, which indicate station water depth (m), sediment depth (cm), and portion of macrofaunal body analyzed (w = whole specimen, g = gut, b = body). Bodies and guts of the same specimens are marked by the same symbols. All sediment 16S rRNA gene sequence data are from Han et al. (2020).
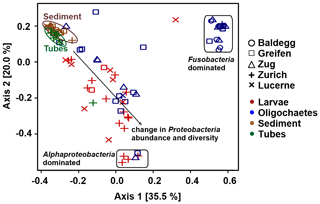
Figure 7PCoA analysis of bacterial community structure at the order level using Bray–Curtis distances. All sediment 16S rRNA gene sequence data are from Han et al. (2020).
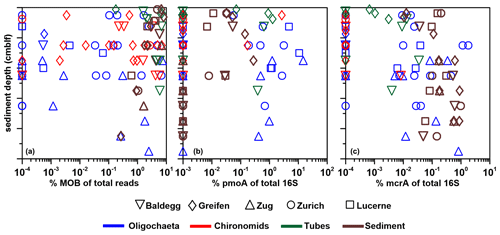
Figure 8Ratios (expressed in %) of bacterial (BAC) 16S rRNA gene copy numbers (left panel), mcrA copy numbers (middle panel), and pmoA copy numbers (right panel) to total 16S rRNA gene copy numbers (sum of bacterial and archaeal 16S rRNA gene copy numbers). The three x axes differ in ranges and scales (linear and log). All sediment 16S rRNA gene values are from Han et al. (2020). Values on the lower limit of the x axis in the middle and right panels indicate samples in which mcrA or pmoA were below qPCR detection.
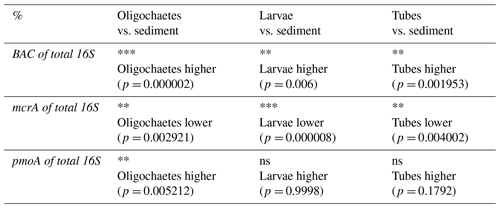
Table 3Results of Wilcoxon sign rank test (one-sided) to examine whether the ratios of bacterial 16S rRNA gene (BAC), mcrA, and pmoA to total 16S rRNA gene copy numbers differ significantly between oligochaete, chironomid larval, and chironomid larval tube samples relative to surrounding sediment (ns = not significant; * = p<0.05; ** = p<0.01; *** = p<0.001), and actual p values can be found in brackets below. Only data were included for which matching values existed from the same sediment depth (±2 cm).
3.2 Macrofaunal community structure and diversity across lakes
Oligochaetes and chironomid larvae were assigned to 9 and 14 different taxonomic groups, respectively (Fig. 4; for station-specific data, see Fig. S2 in the Supplement). All oligochaetes belong to the family Naididae (Syn. Tubificidae) and all chironomid larvae to the family Chironomidae. Two oligochaete morphotypes, Tubificidae + bristles and Tubificidae − bristles, could not be assigned to a known genus.
For Oligochaete group overlap between lakes, four of the nine groups (Tubificidae + bristles, Tubificidae − bristles, P. hammoniensis, L. hoffmeisteri) occur in four of the five lakes. E. velutinus (Lake Zurich), L. profundicula (Lake Baldegg), and P. vejdovskyi (Lake Lucerne) were the only species that were only found in one lake. Comparing the dominant oligochaete groups reveals the dominance of uncharacterized Tubificidae (+ bristles) in Lake Zurich, Lake Zug, and Lake Greifen but very different communities in Lake Baldegg, which is dominated by uncharacterized Tubificidae (− bristles) and L. hoffmeisteri. All identified tubificids except E. velutinus are subsurface deposit feeders that are believed to mainly feed on sedimentary bacteria, whereas E. velutinus is a surface deposit feeder (Table S6 in the Supplement).
Chironomid larval communities in Lake Zurich and Lake Lucerne share many members, but the dominant groups only partially overlap. Lake Zurich sediment is dominated by Micropsectra sp., Tanytarsus sp., Chironomus riparius, Chironomus piger gr., and Sergentia coracina, whereas Lake Lucerne is dominated by Procladius sp., Micropsectra sp., Macropelopia fehlmanni Kieffer 1911, Tanytarsus sp., and S. coracina. Micropsectra sp., Tanytarsus sp., and S. coracina are mainly sedimentary detritus feeders, whereas Chironomus riparius and C. piger gr. are known to mainly filter feed. Both Procladius sp. and M. fehlmanni are predators (Table S6 in the Supplement).
3.3 C isotope composition of macrofauna and bulk C pools
Average C isotope compositions of macrofaunal specimens are displayed with those of the potential C sources methane, TOC, and DOC in Fig. 5 (for depth profiles, see Fig. S3 in the Supplement). Macrofaunal values are lowest in Lake Baldegg (oligochaetes: %, N= 14; larvae: %, N= 4) and Lake Greifen (oligochaetes: %, N= 12; no larvae found) and highest in Lake Lucerne (oligochaetes: %, N= 2; larvae: %, N= 24) and Lake Zurich (oligochaetes: %, N= 5; larvae: %, N= 24). There was no apparent trend between δ13C values of macrofauna and sediment depth (Fig. S3 in the Supplement).
Average δ13C-methane values are in all cases ∼35 % to 50 % more negative than those of macrofauna. The most negative methane values are present in Lake Lucerne ( %, N= 18) and Lake Zurich ( %, N= 25), followed by Lake Baldegg ( %, N= 20), Lake Greifen ( %, N= 21), and Lake Zug ( %, N= 23). All stations except the middle station in Lake Baldegg have 13C-methane increases indicative of methane oxidation in surface layers (Fig. S3 in the Supplement).
The δ13C values of TOC are much closer to those of macrofauna (Fig. 5; Fig. S3 in the Supplement), with averages ranging from equal (Lake Zurich) to ∼5 % higher (Lake Baldegg). The lowest average δ13C-TOC was measured in Lake Greifen ( %, N= 35), followed by Lake Baldegg ( %, N= 37), Lake Zurich ( %, N= 29), Lake Zug ( %, N= 35), and Lake Lucerne ( %, N= 32). Isotopic values of TOC increase by 4 %–6 % with sediment depth at all sites (Fig. S3 in the Supplement). Despite the small differences between δ13C-TOC and δ13C-macrofauna, δ13C-TOC values are significantly higher than those of oligochaetes and larvae in all lakes except Lake Zurich (Fig. 5). Average δ13C-DOC is slightly higher than δ13C-TOC in all lakes and significantly higher than the δ13C of macrofaunal biomass (Fig. 5). Additional analyses on water column algal material and algal bloom layers in sediment (Fig. S3 and Table S4 in the Supplement) suggest δ13C values similar to those of TOC.
3.4 Average contributions of methane-derived carbon and TOC to macrofaunal biomass C
A two end-member mixing model suggests that on average ≥88 % of macrofaunal biomass-C can be explained with assimilation of detrital organic C (TOC) (Table 2). By contrast, methane-derived carbon accounts for ≤12.1 % or ≤6.3 % of biomass-C depending on the assumed isotopic fractionation factor during aerobic methane oxidation (for further details, see Table 1 caption). Chironomid larvae and oligochaetes from the same lakes have highly similar average methane-derived carbon contributions to biomass. Consistent with past studies (Hershey et al., 2006; Jones and Grey, 2011), the contribution of methane-derived carbon to macrofaunal biomass increases with trophic state, with the lowest contributions in Lake Zurich and Lake Lucerne and highest contributions in Lake Baldegg, followed by Lake Zug and Lake Greifen.
3.5 Microbial communities of macrofauna, larval tubes, and surrounding sediments
To investigate the nature of macrofauna–microbiota associations, e.g., with respect to microbial gardening or grazing of methane-cycling microorganisms or symbiotic relationships, we studied 16S rRNA gene sequences of macrofauna (whole organisms, guts, residual body without guts) and chironomid larval tubes and compared these to those in surrounding sediments (Fig. 6).
3.5.1 Bacteria
Sediment and tube samples share similar bacterial communities across all lakes, stations, and sediment depths (Fig. 6). Both sample types are dominated by β-, δ- and γ-Proteobacteria, Chloroflexi (mainly Anaerolineae), Acidobacteria, Bacteroidetes (dominated by Sphingobacteriia), Planctomycetes, and Verrucomicrobia. Furthermore, sediments and tubes from Lake Zurich and Lake Lucerne share elevated fractions of Nitrospirae. Conspicuous differences are the higher fractions of δ-Proteobacteria in sediments and of Chloroflexi, Actinobacteria, Gemmatimonadetes, and Ignavibacteriae in tubes, as well as the virtual absence of Aminicenantes in tubes. By comparison, chironomid larvae and oligochaetes have very different bacterial communities, which moreover vary greatly between and within both macrofaunal groups.
Depending on the specimens, bacterial communities of chironomid larvae are dominated by γ-, β-, and α-Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, and/or Fusobacteria. Many larval specimens are dominated (>50 % of reads) by a single group of α-, β-, or γ-Proteobacteria or Firmicutes, and guts of two specimens from Lake Lucerne contain ≥99 % γ-Proteobacteria. With respect to dominant groups or ZOTUs, there is no clear trend in relation to lake, trophic state, or water depth. Yet, gut, and to a lesser extent body, bacterial communities from the same samples are sometimes highly similar. Furthermore, bacterial communities in guts often differ clearly from those in the remaining body. For instance, Firmicutes in several specimens dominate larval guts but are virtually absent from the rest of the body. By contrast, the fractions of α- and β-Proteobacteria are often lower in guts than the remaining body. Compared to tubes, chironomid larvae generally have lower abundances of Chloroflexi (nearly absent), Verrucomicrobia, Gemmatimonadetes, Nitrospirae, and/or Ignavibacteria.
Bacterial communities of oligochaetes are also variable and differ clearly from those in chironomid larvae. As for chironomid larvae, these bacterial communities do not follow clear trends related to lake, trophic state, or water depth. About half of all specimens are strongly dominated (≥80 % of 16S reads) by Fusobacteria (Fusobacteriales), a phylum that accounts for on average only 0.01±0.02 % of total 16S reads in sediment samples and was only detected in ∼20 % of larval specimens. Several other oligochaete specimens are dominated (>50 %) by single groups of α-, β-, δ-, and ε-Proteobacteria, or Parcubacteria, or have elevated relative abundances of Spirochaetae or Cyanobacteria. Most phyla that are abundant in sediment and/or larval tubes (Chloroflexi, Acidobacteria, Gemmatimonadetes, Nitrospirae, Verrucomicrobiae, Aminicenantes) are less common or nearly absent from oligochaetes. Unlike chironomid larvae, no systematic phylogenetic differences between guts and the rest of the body were detected in oligochaetes. This could, however, be due to the greater difficulty of separating guts from the rest of the body in oligochaetes.
Ordination plots based on PCoA at the order level (Fig. 7) and at the phylum, class, family, and genus levels (Fig. S5 in the Supplement) confirm the trends observed in Fig. 6. Sediment and tube samples from all lakes and sediment depths are highly similar and form tight clusters, which only become separated at the order level and below. Chironomid larvae and oligochaetes are phylogenetically very different from sediments and tubes and phylogenetically highly heterogeneous due to the dominance of Fusobacteria or α-Proteobacteria or the varying relative abundances of diverse proteobacterial classes and orders.
3.5.2 Archaea
Archaea only account for low percentages (<10 %) of prokaryotic 16S rRNA gene sequences in chironomid larvae, larval tubes, and oligochaetes and were even below detection in 69 % of chironomid larval and 39 % of oligochaete samples analyzed (Fig. S4 in the Supplement; also see following section). Yet, distinct trends are evident. Larval tubes have a lower diversity than sediments, being dominated by Woese-, Pace-, and Thaumarchaeota and to a lesser degree Diapherotrites. In sediments, Eury- and Bathyarchaeota were additionally present in high percentages along with low percentages of Altiarchaeales, Lokiarchaeota, and an unclassified phylum-level cluster of Asgardarchaeota. The archaeal community of larvae was highly variable and dominated by Pace-, Eury- and Woesearchaeota, with typically only one to two phyla present per sample. The oligochaete archaeal community was more diverse and dominated by essentially the same groups as sediments, i.e. Woese-, Pace-, Bathy-, Eury-, and/or Thaumarchaeota and to a lesser degree Lokiarchaeota, Altiarchaeales, and Diapherotrites.
3.6 Abundance analysis of Bacteria, Archaea, and functional genes related to methane cycling
To further investigate potential interactions between macrofauna and microorganisms in general, and methane-cycling microorganisms in particular, we compared the contributions of Bacteria, methane-cycling archaea, and methane-oxidizing bacteria across sample types. Trends related to lake trophic state and gardening of or preferential grazing on methane-cycling microorganisms are largely absent, but we observe other trends.
Bacteria account for >80 % of total 16S gene copies in all samples (Fig. 8, left panel). Significantly higher proportions are present in oligochaetes, larvae, and tubes relative to sediments (Table 3). The contribution of Bacteria decreases from 94 %–98 % in surface sediments to 82 %–86 % below 12 cmblf. By comparison, Bacteria contribute ≥99 % in most macrofauna samples. The lowest bacterial contributions are ∼98 % in chironomid larvae, 90 % in oligochaetes, and 96 % in tubes.
In the vast majority of samples, mcrA gene copy numbers are ≥100 times lower than total 16S rRNA gene copy numbers (range: below the detection limit of ∼0.0001 % to 2 %) (Fig. 8, middle panel), suggesting very low contributions of methanogenic and/or anaerobic methanotrophic archaea. The mcrA contributions are significantly higher in sediments compared to oligochaetes, larvae, and tubes (Table 2) and are even below qPCR detection in all but one larval specimen. While the contribution of mcrA increases with depth in larval tubes, oligochaetes and sediments show no depth-related trends. The 16S rRNA genes of methane-cycling Archaea were found in sediments (mainly Methanobacteria and M. fastidiosa) and at very low read numbers in a few tubes (M. fastidiosa) and oligochaetes (M. fastidiosa, M. peredens) but not in larvae.
The pmoA contributions range from below detection ( %) to ∼15 % (Fig. 8, right panel) and are – compared to sediments – significantly elevated in oligochaetes but not in larval specimens or larval tubes (Table 3). This suggests the potential for preferential grazing by, or elevated populations of symbiotic aerobic methanotrophic bacteria within, oligochaetes. Nonetheless, it is worth mentioning that the median calculated pmoA percentage in oligochaetes was only ∼1 % and that based on the maximum calculated value of 15 % methane-oxidizing bacteria in no case dominated oligochaete bacterial communities. As for mcrA, pmoA was only detected in very few (2) larval samples. While pmoA contributions decrease with depth in sediments, there is no clear depth trend in oligochaete or larval tube samples. The 16S rRNA gene sequences indicate that all methane-oxidizing bacteria are γ-Proteobacteria, dominated by Crenothrix (Methylococcales). Crenothrix are moreover the only methane-oxidizing bacterium detected in oligochaetes, whereas low read percentages of Methylococcaceae (Methylobacter, Methylocaldum, Methylococcus, and Methyloparacoccus) were detected in larvae, larval tubes, and sediments. In addition, the denitrifying methanotroph Methylomirabilis (candidate phylum NC10) was detected in low read numbers in several tube and sediment samples (mostly from Lake Lucerne). Despite the significantly higher calculated abundance of methane-oxidizing bacteria in oligochaetes based on ratios of pmoA to total 16S rRNA gene copy numbers, we did not detect significantly different 16S read percentages between larvae, tubes, oligochaetes, or sediments (data not shown).
Methane has been indicated as an important C source to lacustrine sedimentary macrofauna (Kankaala et al., 2006; Deines et al., 2007a; Jones et al., 2008; Jones and Grey, 2011). Yet, open questions remain regarding the conditions under which this methane-derived carbon is an important C source or how it is incorporated into macrofaunal biomass. We investigate these questions by analyzing macrofaunal community structure, isotopic compositions of macrofauna and possible C sources, and microbial community structure across five temperate lakes with widely differing trophic states.
We observe a clear macrofaunal community shift, with oligochaetes dominating eutrophic lakes, chironomid larvae dominating the oligotrophic lake, and similar abundances of both in the mesotrophic lake (Fig. 2). Maximum abundances of oligochaetes are higher than those of chironomid larvae, and oligochaetes extend deeper into sediments than chironomid larvae, matching the different feeding behaviors of the two groups (Fig. 3). Taxonomic analyses reveal overlaps but also clear differences in oligochaete and chironomid larval communities between lakes (Fig. 4).
While chironomid communities vary strongly with water depth in the same lakes, oligochaete communities are more similar across different locations within the same lake. This suggests that chironomid larval and oligochaete communities are controlled by different environmental factors.
Comparing 13C isotopic compositions, 13C-methane is always far more negative (−35 to −50 %), while 13C-TOC is similar or slightly enriched (+0.3 to +5.2 %) relative to macrofaunal biomass. This suggests that detrital organic matter is the main C source of macrofauna (Fig. 5). Estimated contributions of methane-derived carbon range from statistically insignificant to at most 12 % and increase with trophic state (Table 2). Despite differences in feeding behavior and environmental drivers of their species compositions, the calculated contribution of methane-derived carbon is highly similar across chironomid larval and oligochaete specimens from the same lakes, suggesting an important role of lake-specific variables.
Bacterial communities of macrofauna differ clearly from those in chironomid tubes or sediments. The majority of reads in many macrofaunal specimens belong to single ZOTUs, implying potential symbiotic relations with their hosts (Figs. 6 and 7; discussed in detail later). Consistent with the calculated minor contributions of methane-derived C to macrofaunal biomass, pmoA copy numbers indicate that methane-oxidizing bacteria are minor, yet significant, components of bacterial communities in numerous macrofaunal specimens (∼1 %–10 %; Fig. 8, right panel; Table 3). This is not the case for methane-cycling archaea, whose contribution, based on mcrA copy numbers, was always small (≤1 %) and significantly lower in oligochaetes, chironomid larvae, and chironomid larval tubes than in surrounding sediment (Fig. 8, middle panel; Table 3).
In the following sections, we discuss in detail the potential drivers of macrofaunal community structure, the likely carbon sources of macrofauna, and the potential trophic roles of observed (endo-)symbiotic bacteria in their macrofaunal hosts.
4.1 Abundance and taxonomy of macrofauna along trophic state
Oligochaete abundances follow the environmental index proposed previously by Milbrink (1983) which predicts a strong rise in worm abundance with increasing trophic state. Chironomid abundances are also within the range previously reported for lakes (Mousavi, 2002). While chironomid larvae show typical depth distributions (e.g., Panis et al., 1996), oligochaetes have unusually deep ranges, with high abundances to 10–14 cm in eutrophic lakes. By contrast, most publications report that oligochaetes are mainly present at 2–8 cm sediment depth (reviewed in McCall and Tevesz, 1982).
The observed shift in dominance from chironomid larvae to tubificids with increasing trophic state (Fig. 2, Fig. 3) matches past studies reporting the dominance of oligochaetes in eutrophic lakes (Saether, 1980; Lang, 1985; Timm, 1996; Bürgi and Stadelmann, 2002) and changes from chironomid-larva- to oligochaete-dominated communities as the first signs of eutrophication (Saether, 1979). This dominance of oligochaetes in eutrophic lakes is possibly related to an overall higher tolerance of low O2 conditions as many oligochaetes feed in anoxic parts of sediments (McCall and Tevesz, 1982) and efficiently exchange gases through their body walls (Martin et al., 2008). Longer survivorship of anoxic conditions among oligochaetes is also possible (Hamburger et al., 1998), though anaerobic respiration and tolerance of extended anoxic periods is also known for certain species of chironomid larvae (Pinder, 1995). Additional reasons could be the superior ability of oligochaetes to exploit high organic matter supplies or that deeper burrows of oligochaetes provide better protection from benthic predators, such as bottom-feeding fish, which are abundant in eutrophic lakes (Scheffer et al., 1993).
While most oligochaete specimens could only be classified to the family level – Tubificidae (+bristles); Tubificidae (− bristles); Fig. 4; Table S7 in the Supplement – distributions of those that were taxonomically classifiable to the species level match published distributions. On one hand, subsurface deposit feeders known to rely on bacteria and algae as food sources dominated eutrophic lakes. L. hoffmeisteri, an indicator species of eu- to hypertrophic lakes (Brinkhurst, 1982), occurs in high abundances in Lake Baldegg (Table S7 in the Supplement). P. hammoniensis and T. tubifex, which frequently co-occur in high abundances in mesotrophic to eutrophic lakes (Lang, 1990; Timm, 1996), dominate Lake Zurich, Lake Zug, and Lake Greifen. On the other hand, surface-deposit-feeding E. velutinus, which indicates oligo- to mesotrophic conditions (Martin et al., 2008), was only found in Lake Zurich.
Even though many tubificids are subsurface conveyor feeders, the lakes investigated show little evidence of sediment mixing. We observed clear laminations at the deep station in Lake Baldegg and the deep and middle station in Lake Greifen in sediments that were being deposited until the mid-1980s and ∼2010, respectively (Fig. 3, Fig. S7 in the Supplement; Fiskal et al., 2019), so until the onset of artificial water column mixing and oxygenation in these lakes (Lake Baldegg in 1984 and Lake Greifen in 2009; Fiskal et al., 2019). While the subsequent disappearance of laminae suggests rapid re-colonization by macrofauna, it appears that mixing has remained limited to surface sediments even though burrows of tubificids extend far into laminated layers. Depth profiles of radionuclides confirm this interpretation and even indicate minimal sediment mixing in the presence of macrofauna (Fig. S7 in the Supplement). Independent of faunal presence, 137Cs peaks that match the 1986 (Chernobyl) and 1963 (bomb test) time markers, and clear decreases from the top 2 cm downward, are present at all stations. These findings contrast with the rapid sediment homogenization to 10 cm by tubificids in the laboratory (Fisher et al., 1980; Matisoff et al., 1999) and homogeneous radionuclide profiles to 6 cm in tubificid-dominated natural lake sediments (Robbins et al., 1977; Krezoski et al., 1978). Similar to tubificids, chironomid larval communities change in relation to trophic state (Fig. 4; Table S7 in the Supplement). Large free-living and predatory larvae account for half of the specimens in Lake Lucerne, whereas tube-building herbivorous, surface detritus-feeding, and gardening larvae dominate Lake Zurich and the small sample sizes in eutrophic lakes. The shift in diet at higher trophic levels matches the higher input of algae and algal detritus (Fiskal et al., 2019), whereas the potential increase in microbial gardening matches observed increases in gardening by C. riparius and other Chironomus spp. under hypoxic or eutrophic conditions (Stief et al., 2005; Yasuno et al., 2013). By contrast, the reasons for the high abundances of predatory larvae in Lake Lucerne are unclear. Possible reasons are the low hypoxia tolerance of large predatory Macropelopia and Procladius spp. (Hamburger et al., 1998; Brodersen et al., 2008), higher availability of zooplankton food in oligotrophic lakes (Jeppesen et al., 1990; Jeppesen et al., 1999), and/or stronger predation pressure in mesotrophic and eutrophic lakes, which often have high populations of bottom-feeding fish (Scheffer et al., 1993).
4.2 Carbon sources of lake sedimentary macrofauna
Similar to previous studies (e.g., Grey et al., 2004; Jones et al., 2008) we calculate an increase in the contribution of methane-derived carbon with increasing trophic state (Fig. 5; Table 2). Yet, this contribution is at most 12 %, even in the highly eutrophic lakes. Other studies have estimated methane-derived carbon contributions of >40 % for chironomid larvae in eutrophic lakes (e.g., Deines and Grey, 2006; Eller et al., 2007; Jones et al., 2008) and reported strong δ13C depletions in oligochaete specimens from profundal sediment (Premke et al., 2010). Yet, minor contributions of methane-derived carbon to the biomass of benthic invertebrates are not new. A survey of 87 lakes suggested that marked 13C depletions were only present in chironomid larvae from lakes with seasonal stratification and bottom water anoxia (Jones et al., 2008). Moreover, the limited published δ13C data on lake oligochaetes are mostly similar to those of TOC (Kiyashko et al., 2001; Premke et al., 2010).
In support of C-isotopic interpretations, DNA-based analyses indicate that neither methane-oxidizing bacteria nor methanogens are dominant microorganisms in surface sediments or chironomid larval tubes. Thus, strong enrichment or gardening of methane-oxidizing bacteria or methanogens as observed elsewhere in chironomid tubes (e.g., Kajan and Frenzel, 1999; Kelly et al., 2004) or surface sediments (e.g., Eller et al., 2005; Deines et al., 2007a) is absent for reasons that are unclear. Despite being artificially oxygenated, bottom water in Lake Baldegg and Lake Greifen experiences seasonally low O2 conditions (0.5–4 mg L−1) or hypoxic conditions (<0.5 mg L−1), respectively (Fiskal et al., 2019). These values are within or below the seasonal O2 threshold (2–4 mg L−1) that is characteristic of lakes with marked 13C depletions in chironomid biomass (Jones et al., 2008). Jones et al. (2008) argued that the contribution of methane-derived carbon increases inversely with the depth of the oxic–anoxic interface. In June 2016, this interface was ≤1 mm at all stations in Lake Baldegg and ≤2 mm at those in Lake Greifen, while methanogenesis occurred in the top 1 cm of sediment (Fiskal et al., 2019). Thus, conditions were potentially well-suited for the strong enrichment of methane-cycling microorganisms. It is possible that the growth of methane-oxidizing bacteria is mainly promoted at narrow oxic–anoxic (high O2-methane) interfaces produced by ventilating and tube-building chironomid larvae (Brune et al., 2000). Tubificids, which dominated our eutrophic lakes, do not produce such stable oxic–anoxic interfaces and also perform less burrow ventilation than chironomid larvae (Gautreau et al., 2020 and references within). Yet, the fact that all three identified larvae from Lake Baldegg belong to tube-building taxa and that the four isotopically analyzed larvae from this lake only had minor methane-derived carbon contributions suggests that yet unknown factors contribute to the enrichment of methane-oxidizing bacteria by tube-building chironomids in surface sediment.
Instead of methane-derived carbon, our C-isotopic data indicate that algal or detrital organic carbon, or microorganisms that have assimilated the isotopic signatures of algal or detrital organic carbon, is the main food source of dominant macrofauna (Table 2). Rather than methane-derived carbon, selective feeding on isotopically depleted subportions of the TOC pool could even, in principle, explain the minor isotopic depletions of oligochaete and chironomid larval biomass in eutrophic lakes. Yet, our limited data on algal bloom layers in sediments and phytoplankton from overlying water indicate similar 13C values relative to TOC (Fig. S5 in the Supplement). Preferential feeding on organic C from surface sediments, which in many cases has the lowest C-isotopic values, or isotopic fractionations during C-assimilation and biosynthesis are also not plausible. As bottom-up conveyor feeders, tubificids feed mostly at several centimeters depth (McCall and Tevesz, 1982), and C-isotopic fractionation during biosynthesis of bulk animal biomass is typically low (Fry and Sherr, 1989).
4.3 Potential diet and host–microorganism interactions in tubificid worms
Minimal sediment reworking and deep sedimentary distributions of tubificids suggest that shallow subsurface deposit feeding may not be the main dietary mode of these worms in the lakes studied, raising questions concerning their main foraging strategy. One possibility is that oligochaetes selectively graze on microbial biofilms inhabiting the walls of their deep and extensive gallery-type burrow networks. Under this scenario one might expect large amounts of DNA of sediment microorganisms in oligochaete intestines. This is not the case, however, suggesting that grazed communities are very different from those in sediments or their DNA is rapidly digested. Another foraging strategy may not involve ingestion via the oral cavity but diffusive uptake. T. tubifex can actively take up short-chain organic acids, such as acetate and propionate, through their body wall (Hipp et al., 1985; Sedlmeier and Hoffmann, 1989). The subsequent respiration of these organic acids can account for up to 40 % of T. tubifex energy turnover (Hipp et al., 1986). Other species of tubificids take up amino acids through the body wall (Brinkhurst and Chua, 1969). Tubificid body walls are also permeable to dissolved gases, which is why tubificids acquire O2 by undulating movements of their tail ends in oxic water above sediments (Brinkhurst, 1996). Permeability to gases could also provide energy if, for example, methane or H2 diffusing from pore water into worms supports symbiotic microorganisms.
Matching the slight increase in methane-derived carbon in oligochaetes from eutrophic lakes, we observe higher contributions of pmoA in oligochaetes compared to surrounding sediment. Assuming that these pmoA belong to living methane-oxidizing bacteria, movement of oligochaetes between methane-rich, deeper layers and the oxic sediment surface could favor their growth and result in an endosymbiotic relationship. The potential for annelid hindguts to make excellent microbial habitats was previously demonstrated in the polychaete Abarenicola vagabunda (Plante et al., 1989). How this methane-derived carbon would be assimilated is unclear, however. Potential mechanisms include uptake of organic intermediates of methane oxidation, e.g., methanol, through the hindgut or ingestion of faeces that are enriched in methane-oxidizing bacteria.
The overwhelming majority of microbial DNA from oligochaetes, however, belongs to Bacteria, often single ZOTUs, which are not linked to methane oxidation. In 22 of the 30 specimens sequenced, a single ZOTU accounted for >50 % of the total reads (Table S8 in the Supplement). In 15 specimens, this dominant ZOTU belonged to a single genus-level cluster of unclassified Fusobacteriaceae (Fusobacteriaceae Cluster I) that was previously found in earthworm and aquatic vertebrate intestines, anaerobic sediments, bioreactors, soil, and diverse water samples (Fig. S7A in the Supplement). The high percentages of this cluster are striking considering that Fusobacteriaceae account for on average only 0.01±0.02 % of total 16S reads in the surrounding sediments. All cultivated members of Fusobacteriaceae are anaerobes that fermentatively degrade polymeric organic compounds, in particular proteins and carbohydrates, with acetate, butyrate, and other short-chain organic acids as main end products (Olsen, 2014). Given previous evidence for the preference of proteinaceous organic matter by tubificids (de Valk et al., 2017), these Fusobacteriaceae could be primary degraders of proteins within the digestive tracts of oligochaetes. This relationship could be mutually beneficial, commensal, or parasitic. A mutually beneficial relationship could entail symbionts gaining energy by fermenting proteins that are not digestible by the host and through the host respiring the resulting fermentation products.
The remaining seven dominant ZOTUs belong to the phyla Proteobacteria (α, β, and ε classes), Bacteroidetes, and Parcubacteria (Fig. S8, Table S8 in the Supplement). ZOTU18 falls into the anaerobic ε-proteobacterial genus Wolinella (order Campylobacterales), isolates of which use H2 or formate as electron donors and fumarate and nitrate as electron acceptors (Tanner and Paster, 1992). Succinate is the main end product of fumarate reduction by Wolinella and could benefit hosts under low O2 conditions given that succinate is the main intermediate during anaerobic metabolism of tubificids (Seuß et al., 1983). ZOTU8 falls into the facultatively aerobic β-proteobacterial genus Deefgea (order Neisseriales), members of which ferment carbohydrates to organic acids (Stackebrandt et al., 2007) and could benefit hosts as proposed for Fusobacteriaceae. The α-proteobacterial ZOTU4 falls into the family Holosporaceae, members of which are obligately intracellular, potentially parasitic symbionts of ciliates (Santos and Massard, 2014). ZOTU4 could derive from commensal ciliates, which often inhabit guts of freshwater oligochaetes (Falls, 1972). Alternatively, given the high percentage of 16S reads in one specimen (93 %), a novel form of (intracellular) symbiosis with tubificids cannot be discounted. Similarly unclear is the host relationship with ZOTU199, which belongs to the candidate phylum Parcubacteria of the Candidate Phyla Radiation (Brown et al., 2015). Members of this phylum have been retrieved from diverse, mostly anoxic habitats, have genes linked to carbohydrate fermentation, and have been implicated in ectosymbiotic or parasitic lifestyles (Wrighton et al., 2012; Nelson and Stegen, 2015). The remaining ZOTUs fall into an unclassified genus-level subcluster of β-Proteobacteria (ZOTU6; order Rhodocyclales) and an unclassified order-level cluster of ε-Proteobacteria (ZOTU9). Based on existing knowledge, it is not possible to infer the potential roles of these ZOTUs within their hosts.
4.4 Potential host–microorganism interactions in chironomid larvae
Similar to tubificids, most chironomid larvae (12 of 19 sequenced specimens) are dominated by single ZOTUs (Table S8 in the Supplement). Interestingly, more specimens are dominated by single ZOTUs in Lake Lucerne (9 of 10) than in Lake Zurich (three of seven) or Lake Baldegg (zero of two), suggesting that the frequency and/or importance of these associations is linked to trophic state. These single dominant ZOTUs are mostly Proteobacteria (10 of 12; α, β, and γ classes). In addition, single specimens were dominated by the same unclassified Fusobacteriaceae (Fusobacteriaceae Cluster I) that dominate tubificids and an unclassified sister group of Bacteroides, Bacteroidetes), which we call “Unclassified Wastewater and Gut Group” based on reported occurrences.
Two proteobacterial groups most commonly dominate chironomid larvae. ZOTU2 of the α-proteobacterial genus Wolbachia (Rickettsiales) dominates four specimens. Members of this genus are widespread intracellular symbionts of insects whose relationships with their hosts range from parasitic to mutualistic (Correa and Ballard, 2016), though, to our knowledge, dietary contributions have not been demonstrated. ZOTU3 and ZOTU21 of the γ-proteobacterial genus Aeromonas dominate three specimens. Members of this facultatively anaerobic genus are widespread in aquatic habitats (Huys, 2014) and were previously found in aquatic invertebrates, including chironomid larvae (Eller et al., 2007). Aeromonads can ferment carbohydrates to organic acids, which might supplement the diet of chironomid larvae, but they have also been shown to degrade the egg masses of chironomids (Senderovich et al., 2008). Similar functions, ranging from mutualistic to detrimental, are likely for the γ-proteobacterial genus Serratia (γ-Proteobacteria) and an unclassified cluster of Moraxellaceae (Pseudomonadales) and for the Unclassified Wastewater and Gut Group. ZOTUs of these groups each dominate one larval specimen (ZOTU11, ZOTU26, and ZOTU28, respectively). All three groups degrade carbohydrates anaerobically (Serratia, Bacteroidetes) or aerobically/facultatively anaerobically (Moraxellaceae) to organic acids, which may provide energy to larvae but can also be pathogenic or mutualistic in ways unrelated to diet (Grimont and Grimont, 2006; Sabri et al., 2011; Teixeira and Merquior, 2014; Wexler, 2014). The remaining ZOTUs belong to unclassified genus-level subclusters of β-proteobacterial Rhodocyclales (ZOTU6) and Burkholderiales (ZOTU12). Due to the very diverse ecophysiologies of Rhodocyclales and Burkholderiales the potential roles of these ZOTUs within their hosts are highly uncertain.
Our study indicates clear changes in lacustrine sedimentary macrofaunal communities with increasing trophic state, including a shift in dominance from chironomid larvae to tubificid oligochaetes. Carbon isotopic and genetic analyses show that, independent of faunal group or trophic state, detritus-derived organic carbon rather than methane-derived carbon is the main carbon source of these animals. Yet, the exact carbon sources remain unclear and may include actual detritus, detrital carbon-assimilating microorganisms, and/or waste products of microbial detritus degradation. Thus bacterial symbionts that are abundant within tubificids and chironomids but rare in surrounding sediment could be important. Known carbon sources of these symbionts provide potential clues to predominant tubificid and larval food sources. Given that most tubificid specimens are dominated by known protein-degrading bacteria (Fusobacteriaceae), selective feeding on protein-rich organic matter fractions, such as microbial cells, is likely for these specimens. Similarly, given that half of the dominant ZOTUs in chironomid larvae belong to carbohydrate-degrading taxa (Aeromonas, Serratia, Moraxellaceae, Bacteroidales), preferential feeding on algal detritus in surface sediments is plausible for these chironomid taxa. Though more research is needed, both macrofaunal groups may benefit from their endosymbionts through the production of short-chain organic acids, which can be taken up through the hindgut wall and subsequently used for energy conservation or biosynthesis.
Biogeochemical data will be made available after publication of the manuscript on Pangaea. The sequence data have been deposited at DDBJ/EMBL/GenBank under the accession KDVU00000000. The version described in this paper is the first version, KDVU01000000.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-4369-2021-supplement.
AF, AM, EA, LD, XH, RZ, LL, ND, CJS, SMB, and MAL helped with sample collection and/or measurements. AF, SMB, and MAL substantially contributed to the interpretation of data. AF and MAL wrote the manuscript. MAL designed the study and acquired the funding for the project. All authors commented on and approved the final version of the manuscript.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank the Genetic Diversity Centre (GDC) at ETH Zurich for help with next generation sequencing and analysis. We are thankful to AquaDiptera and AquaLytis for the taxonomic analysis of the macrofauna samples, especially to Susanne Michiels and Ute Michels. We thank Madalina Jaggi for technical support of macrofaunal 13C analysis and Serge Robert for technical support during δ13C-methane measurements.
This research has been supported by the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant no. 205321_163371).
This paper was edited by Aninda Mazumdar and reviewed by two anonymous referees.
Anderson, N. J., Dietz, R. D., and Engstrom, D. R.: Land-use change, not climate, controls organic carbon burial in lakes, P. Roy. Soc. B-Biol. Sci., 280, ARTN 20131278 https://doi.org/10.1098/rspb.2013.1278, 2013.
Anderson, N. J., Bennion, H., and Lotter, A. F.: Lake eutrophication and its implications for organic carbon sequestration in Europe, Global Change Biol., 20, 2741–2751, https://doi.org/10.1111/gcb.12584, 2014.
Andersson, G., Graneli, W., and Stenson, J.: The Influence of Animals on Phosphorus Cycling in Lake Ecosystems, Hydrobiologia, 170, 267–284, https://doi.org/10.1007/Bf00024909, 1988.
Armitage, P. D., Pardo, I., and Brown, A.: Temporal Constancy of Faunal Assemblages in Mesohabitats – Application to Management, Arch. Hydrobiol., 133, 367–387, 1995.
Aston, R.: Tubificids and water quality: a review, Environ. Pollut., 5, 1–10, 1973.
Baranov, V., Lewandowski, J., Romeijn, P., Singer, G., and Krause, S.: Effects of bioirrigation of non-biting midges (Diptera: Chironomidae) on lake sediment respiration, Sci. Rep.-UK, 6, ARTN 27329 https://doi.org/10.1038/srep27329, 2016.
Bastviken, D., Cole, J., Pace, M., and Tranvik, L.: Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate, Global Biogeochem. Cy., 18, Artn Gb4009 https://doi.org/10.1029/2004gb002238, 2004.
Brinkhurst, R. O. and Chua, K. E.: Preliminary investigation of the exploitation of some potential nutritional resources by three sympatric tubificid oligochaetes, J. Fis. Res. Board Can, 26, 2659–2668, 1969.
Brinkhurst, R. O.: Evolution in the Annelida, Can. J. Zool., 60, 1043–1059, https://doi.org/10.1139/z82-145, 1982.
Brinkhurst, R. O.: On the role of tubificid oligochaetes in relation to fish disease with special reference to the Myxozoa, Annual Review of Fish Diseases, 6, 29–40, 1996.
Brodersen, K. P., Pedersen, O., Walker, I. R., and Jensen, M. T.: Respiration of midges (Diptera; Chironomidae) in British Columbian lakes: oxy-regulation, temperature and their role as palaeo-indicator, Freshwater Biol., 53, 593–602, 2008.
Brown, C. T., Hug, L. A., Thomas, B. C., Sharon, I., Castelle, C. J., Singh, A., Wilkins, M. J., Wrighton, K. C., Williams, K. H., and Banfield, J. F.: Unusual biology across a group comprising more than 15 % of domain Bacteria, Nature, 523, 208–211, 2015.
Brune, A., Frenzel, P., and Cypionka, H.: Life at the oxic–anoxic interface: microbial activities and adaptations, Fems Microbiol. Rev., 24, 691–710, 2000.
Bürgi, H. and Stadelmann, P.: Change of phytoplankton composition and biodiversity in Lake Sempach before and during restoration, Hydrobiologia, 469, 33–48, 2002.
Bussmann, I.: Methane release through resuspension of littoral sediment, Biogeochemistry, 74, 283–302, 2005.
Chapman, P. M., Farrell, M. A., and Brinkhurst, R. O.: Relative Tolerances of Selected Aquatic Oligochaetes to Individual Pollutants and Environmental-Factors, Aquat. Toxicol., 2, 47–67, https://doi.org/10.1016/0166-445x(82)90005-4, 1982.
Claesson, M. J., O'Sullivan, O., Wang, Q., Nikkila, J., Marchesi, J. R., Smidt, H., de Vos, W. M., Ross, R. P., and O'Toole, P. W.: Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine, PloS ONE, 4, e6669, https://doi.org/10.1371/journal.pone.0006669, 2009.
Correa, C. C. and Ballard, J.: Wolbachia associations with insects: winning or losing against a master manipulator, Front. Ecol. Evol., 3, 153, https://doi.org/10.3389/fevo.2015.00153, 2016.
Davis, R. B.: Tubificids alter profiles of redox potential and pH in profundal lake sediment 1, Limnol. Oceanogr., 19, 342–346, 1974.
de Valk, S., Khadem, A. F., Foreman, C. M., van Lier, J. B., and de Kreuk, M. K.: Physical and biochemical changes in sludge upon Tubifex tubifex predation, Environ. Technol., 38, 1524–1538, 2017.
Dean, W. E. and Gorham, E.: Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands, Geology, 26, 535–538, 1998.
Deines, P. and Grey, J.: Site-specific methane production and subsequent midge mediation within Esthwaite Water, UK, Arch. Hydrobiol., 167, 317–334, https://doi.org/10.1127/0003-9136/2006/0167-0317, 2006.
Deines, P., Bodelier, P. L. E., and Eller, G.: Methane-derived carbon flows through methane-oxidizing bacteria to higher trophic levels in aquatic systems, Environ. Microbiol., 9, 1126–1134, https://doi.org/10.1111/j.1462-2920.2006.01235.x, 2007a.
Deines, P., Grey, J., Richnow, H. H., and Eller, G.: Linking larval chironomids to methane: seasonal variation of the microbial methane cycle and chironomid delta C-13, Aquat. Microb. Ecol., 46, 273–282, https://doi.org/10.3354/ame046273, 2007b.
DelSontro, T., Boutet, L., St-Pierre, A., del Giorgio, P. A., and Prairie, Y. T.: Methane ebullition and diffusion from northern ponds and lakes regulated by the interaction between temperature and system productivity, Limnol Oceanogr, 61, S62-S77, https://doi.org/10.1002/lno.10335, 2016.
Edgar, R. C. J. B.: UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing, bioRxiv, https://doi.org/10.1101/081257, 2016.
Einsele, G., Yan, J. P., and Hinderer, M.: Atmospheric carbon burial in modern lake basins and its significance for the global carbon budget, Global Planet. Change, 30, 167–195, https://doi.org/10.1016/S0921-8181(01)00105-9, 2001.
Eller, G., Deines, P., Grey, J., Richnow, H. H., and Kruger, M.: Methane cycling in lake sediments and its influence on chironomid larval partial derivative C-13, Fems Microbiol. Ecol., 54, 339–350, https://doi.org/10.1016/j.femsec.2005.04.006, 2005.
Eller, G., Deines, P., and Kruger, M.: Possible sources of methane-derived carbon for chironomid larvae, Aquat. Microbiol. Ecol., 46, 283–293, https://doi.org/10.3354/ame046283, 2007.
Falls, E. Q.: A survey of fresh-water oligochaeta and their commensal ciliates from the Richmond, Virginia area, Master's Theses, Paper 343, 1972.
Figueiredo-Barros, M. P., Caliman, A., Leal, J. J., Bozelli, R. L., Farjalla, V. F., and Esteves, F. A.: Benthic bioturbator enhances CH4 fluxes among aquatic compartments and atmosphere in experimental microcosms, Can. J. Fish. Aquat. Sci., 66, 1649–1657, 2009.
Fisher, J., Lick, W., McCall, P., and Robbins, J.: Vertical mixing of lake sediments by tubificid oligochaetes, J. Geophys. Res.-Oceans, 85, 3997–4006, 1980.
Fiskal, A., Deng, L., Michel, A., Eickenbusch, P., Han, X., Lagostina, L., Zhu, R., Sander, M., Schroth, M. H., Bernasconi, S. M., Dubois, N., and Lever, M. A.: Effects of eutrophication on sedimentary organic carbon cycling in five temperate lakes, Biogeosciences, 16, 3725–3746, https://doi.org/10.5194/bg-16-3725-2019, 2019.
Fry, B. and Sherr, E. B.: δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems, in: Stable isotopes in ecological research, edited by: Rundel, P. W., Ehleringer, J. R., and Nagy K. A., vol 68, Springer, New York, NY, https://doi.org/10.1007/978-1-4612-3498-2_12, 1989.
Gautreau, E., Volatier, L., Nogaro, G., Gouze, E., and Mermillod-Blondin, F.: The influence of bioturbation and water column oxygenation on nutrient recycling in reservoir sediments, Hydrobiologia, 847, 1027–1040, https://doi.org/10.1007/s10750-019-04166-0, 2020.
Gentzel, T., Hershey, A. E., Rublee, P. A., and Whalen, S. C.: Net sediment production of methane, distribution of methanogens and methane-oxidizing bacteria, and utilization of methane-derived carbon in an arctic lake, Inland Waters, 2, 77–88, https://doi.org/10.5268/Iw-2.2.416, 2012.
Grey, J., Kelly, A., Ward, S., Sommerwerk, N., and Jones, R. I.: Seasonal changes in the stable isotope values of lake-dwelling chironomid larvae in relation to feeding and life cycle variability, Freshwater Biol., 49, 681–689, https://doi.org/10.1111/j.1365-2427.2004.01217.x, 2004.
Grimont, F. and Grimont, P.: The genus Serratia, in: Dworkin, M. et al., The Prokaryotes, Vol. 6, Springer Science and Business Media, New York, NY, 2006.
Hamburger, K., Lindegaard, C., Dall, P. C., and Nilson, I.: Strategies of respiration and glycogen metabolism in oligochaetes and chironomids from habitats exposed to different oxygen deficits, SIL Proceedings, 1922–2010, 26, 2070–2075, https://doi.org/10.1080/03680770.1995.11901107, 1998.
Han, X. G., Schubert, C. J., Fiskal, A., Dubois, N., and Lever, M. A.: Eutrophication as a driver of microbial community structure in lake sediments, Environ. Microbiol., 22, 3446–3462, 2020.
Heathcote, A. J. and Downing, J. A.: Impacts of Eutrophication on Carbon Burial in Freshwater Lakes in an Intensively Agricultural Landscape, Ecosystems, 15, 60–70, https://doi.org/10.1007/s10021-011-9488-9, 2012.
Hershey, A. E., Beaty, S., Fortino, K., Kelly, S., Keyse, M., Luecke, C., O'Brien, W. J., and Whalen, S. C.: Stable isotope signatures of benthic invertebrates in arctic lakes indicate limited coupling to pelagic production, Limnol. Oceanogr., 51, 177–188, https://doi.org/10.4319/lo.2006.51.1.0177, 2006.
Hipp, E., Mustafa, T., and Hoffmann, K.: Integumentary uptake of volatile fatty acids by the freshwater oligochaete Tubifex, Naturwissenschaften, 72, 148–149, 1985.
Hipp, E., Bickel, U., Mustafa, T., and Hoffmann, K. H.: Integumentary uptake of acetate and propionate (VFA) by Tubifex sp, a freshwater oligochaete. I I. Role of VFA as nutritional resources and effects of anaerobiosis, J. Exp. Zool., 240, 299–308, 1986.
Holgerson, M. A. and Raymond, P. A.: Large contribution to inland water CO2 and CH4 emissions from very small ponds, Nat. Geosci., 9, 222-U150, https://doi.org/10.1038/Ngeo2654, 2016.
Holker, F., Vanni, M. J., Kuiper, J. J., Meile, C., Grossart, H. P., Stief, P., Adrian, R., Lorke, A., Dellwig, O., Brand, A., Hupfer, M., Mooij, W. M., Nutzmann, G., and Lewandowski, J.: Tube-dwelling invertebrates: tiny ecosystem engineers have large effects in lake ecosystems, Ecol. Monogr., 85, 333–351, https://doi.org/10.1890/14-1160.1, 2015.
Hollander, D. J., Mckenzie, J. A., and Lotenhaven, H.: A 200-Year Sedimentary Record of Progressive Eutrophication in Lake Greifen (Switzerland) – Implications for the Origin of Organic-Carbon-Rich Sediments, Geology, 20, 825–828, https://doi.org/10.1130/0091-7613(1992)020<0825:Aysrop>2.3.Co;2, 1992.
Hupfer, M. and Lewandowski, J.: Oxygen Controls the Phosphorus Release from Lake Sediments – a Long-Lasting Paradigm in Limnology, Int. Rev. Hydrobiol., 93, 415–432, https://doi.org/10.1002/iroh.200711054, 2008.
Hupfer, M., Jordan, S., Herzog, C., Ebeling, C., Ladwig, R., Rothe, M., and Lewandowski, J.: Chironomid larvae enhance phosphorus burial in lake sediments: Insights from long-term and short-term experiments, Sci. Total Environ., 663, 254–264, https://doi.org/10.1016/j.scitotenv.2019.01.274, 2019.
Huys, G.: The Family Aeromonadaceae, The Prokaryotes-Gammaproteobacteria Springer-Verlag, Berlin Heidelberg, 27–57, 2014.
Jeppesen, E., Søndergaard, M., Sortkjær, O., Mortensen, E., and Kristensen, P.: Interactions between phytoplankton, zooplankton and fish in a shallow, hypertrophic lake: a study of phytoplankton collapses in Lake Søbygård, Denmark, in: Trophic Relationships in Inland Waters, Springer, Dordrecht, 149–164, 1990.
Jeppesen, E., Jensen, J. P., Sondergaard, M., Lauridsen, T., Moller, F. P., and Sandby, K.: Changes in nitrogen retention in shallow eutrophic lakes following a decline in density of cyprinids, Arch. Hydrobiol., 142, 129–151, 1998.
Jeppesen, E., Jensen, J. P., Søndergaard, M., and Lauridsen, T.: Trophic dynamics in turbid and clearwater lakes with special emphasis on the role of zooplankton for water clarity, in: Shallow lakes' 98, Springer, Dordrecht, 217–231, 1999.
Jones, R. I., Carter, C. E., Kelly, A., Ward, S., Kelly, D. J., and Grey, J.: Widespread Contribution of Methane-Cycle Bacteria to the Diets of Lake Profundal Chironomid Larvae, Ecology, 89, 857–864, https://doi.org/10.1890/06-2010.1, 2008.
Jones, R. I. and Grey, J.: Biogenic methane in freshwater food webs, Freshwater Biol., 56, 213–229, https://doi.org/10.1111/j.1365-2427.2010.02494.x, 2011.
Kajan, R. and Frenzel, P.: The effect of chironomid larvae on production, oxidation and fluxes of methane in a flooded rice soil, Fems Microbiol. Ecology, 28, 121–129, 1999.
Kankaala, P., Taipale, S., Grey, J., Sonninen, E., Arvola, L., and Jones, R. I.: Experimental delta C-13 evidence for a contribution of methane to pelagic food webs in lakes, Limnol. Oceanogr., 51, 2821–2827, https://doi.org/10.4319/lo.2006.51.6.2821, 2006.
Kankaala, P., Taipale, S., Nykanen, H., and Jones, R. I.: Oxidation, efflux, and isotopic fractionation of methane during autumnal turnover in a polyhumic, boreal lake, J. Geophys. Res.-Biogeo., 112, Artn G02003 https://doi.org/10.1029/2006jg000336, 2007.
Katsev, S., Tsandev, I., L'Heureux, I., and Rancourt, D. G.: Factors controlling long-term phosphorus efflux from lake sediments: Exploratory reactive-transport modeling, Chem. Geol., 234, 127–147, https://doi.org/10.1016/j.chemgeo.2006.05.001, 2006.
Kelly, A., Jones, R., and Grey, J.: Stable isotope analysis provides fresh insights into dietary separation between Chironomus anthracinus and C. plumosus, J. N. Am. Benthol. Soc., 23, 287–296, 2004.
Kiyashko, S. I., Narita, T., and Wada, E.: Contribution of methanotrophs to freshwater macroinvertebrates: evidence from stable isotope ratios, Aquat. Microb. Ecol., 24, 203–207, https://doi.org/10.3354/ame024203, 2001.
Krezoski, J. R., Mozley, S. C., and Rohhins, J. A.: Influence of benthic macroinvertebrates on mixing of profundal sediments in southeastern Lake Huron 1, Limnol. Oceanogr., 23, 1011–1016, 1978.
Kristensen, E., Penha-Lopes, G., Delefosse, M., Valdemarsen, T., Quintana, C. O., and Banta, G. T.: What is bioturbation? The need for a precise definition for fauna in aquatic sciences, Mar. Ecol. Prog. Ser., 446, 285–302, 2012.
Kruger, M., Eller, G., Conrad, R., and Frenzel, P.: Seasonal variation in pathways of CH4 production and in CH4 oxidation in rice fields determined by stable carbon isotopes and specific inhibitors, Global Change Biol., 8, 265–280, https://doi.org/10.1046/j.1365-2486.2002.00476.x, 2002.
Lagauzère, S., Pischedda, L., Cuny, P., Gilbert, F., Stora, G., and Bonzom, J.-M.: Influence of Chironomus riparius (Diptera, Chironomidae) and Tubifex tubifex (Annelida, Oligochaeta) on oxygen uptake by sediments. Consequences of uranium contamination, Environ. Pollut., 157, 1234–1242, 2009.
Lang, C.: Eutrophication of Lake Geneva indicated by the oligochaete communities of the profundal, Hydrobiologia, 126, 237–243, 1985.
Lang, C.: Quantitative relationships between oligochaete communities and phosphorus concentrations in lakes, Freshwater Biol., 24, 327–334, 1990.
Lang, S. Q., Bernasconi, S. M., and Früh-Green, G. L.: Stable isotope analysis of organic carbon in small (µg C) samples and dissolved organic matter using a GasBench preparation device, Rapid Commun. Mass. Sp., 26, 9–16, 2012.
Lever, M. A., Torti, A., Eickenbusch, P., Michaud, A. B., Santl-Temkiv, T., and Jorgensen, B. B.: A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types, Front. Microbiol., 6, UNSP 476 https://doi.org/10.3389/fmicb.2015.00476, 2015.
Lewandowski, J., Laskov, C., and Hupfer, M.: The relationship between Chironomus plumosus burrows and the spatial distribution of pore-water phosphate, iron and ammonium in lake sediments, Freshwater Biol., 52, 331–343, https://doi.org/10.1111/j.1365-2427.2006.01702.x, 2007.
Maerki, M., Muller, B., Dinkel, C., and Wehrli, B.: Mineralization pathways in lake sediments with different oxygen and organic carbon supply, Limnol. Oceanogr., 54, 428–438, https://doi.org/10.4319/lo.2009.54.2.0428, 2009.
Magoč, T. and Salzberg, S. L. J. B.: FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 27, 2957–2963, 2011.
Martin, P., Martinez-Ansemil, E., Pinder, A., Timm, T., and Wetzel, M. J.: Global diversity of oligochaetous clitellates (”Oligochaeta”; Clitellata) in freshwater, Hydrobiologia, 595, 117–127, https://doi.org/10.1007/s10750-007-9009-1, 2008.
Matisoff, G., Wang, X. S., and McCall, P. L.: Biological redistribution of lake sediments by tubificid oligochaetes: Branchiura sowerbyi and Limnodrilus hoffmeisteri/Tubifex tubifex, J. Great Lakes Res., 25, 205–219, https://doi.org/10.1016/S0380-1330(99)70729-X, 1999.
McCall, P. L. and Tevesz, M. J. S.: The Effects of Benthos on Physical Properties of Freshwater Sediments, in: Animal-Sediment Relations: The Biogenic Alteration of Sediments, edited by: McCall, P. L. and Tevesz, M. J. S., Topics in Geobiology, Springer US, Boston, MA, 105–176, 1982.
Mendonça, R., Müller, R. A., Clow, D., Verpoorter, C., Raymond, P., Tranvik, L. J., and Sobek, S.: Organic carbon burial in global lakes and reservoirs, Nat. Commun., 8, 1–7, 2017.
Mermillod-Blondin, F., Nogaro, G., Datry, T., Malard, F., and Gibert, J.: Do tubificid worms influence the fate of organic matter and pollutants in stormwater sediments?, Environ. Pollut., 134, 57–69, https://doi.org/10.1016/j.envpol.2004.07.024, 2005.
Meysman, F. J. R., Middelburg, J. J., and Heip, C. H. R.: Bioturbation: a fresh look at Darwin's last idea, Trends Ecol. Evol., 21, 688–695, https://doi.org/10.1016/j.tree.2006.08.002, 2006.
Milbrink, G.: An improved environmental index based on the relative abundance of oligochaete species, Hydrobiologia, 102, 89–97, 1983.
Mousavi, S. K.: Boreal chironomid communities and their relations to environmental factors – the impact of lake depth, size and acidity, Boreal Environ. Res., 7, 63–75, 2002.
Nelson, W. C. and Stegen, J. C.: The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle, Front. Microbiol., 6, 713, https://doi.org/10.3389/fmicb.2015.00713, 2015.
Nicacio, G. and Juen, L.: Chironomids as indicators in freshwater ecosystems: an assessment of the literature, Insect Conserv. Diver., 8, 393–403, 2015.
Olsen, I.: The Family Fusobacteriaceae, in: The Prokaryotes: Firmicutes and Tenericutes, edited by: Rosenberg, E., DeLong, E. F., Lory, S., Stackebrandt, E., and Thompson, F., Springer, Berlin, Heidelberg, 109–132, 2014.
Panis, L. I., Goddeeris, B., and Verheyen, R.: On the relationship between vertical microdistribution and adaptations to oxygen stress in littoral Chironomidae (Diptera), Hydrobiologia, 318, 61–67, 1996.
Pelegri, S. P., Nielsen, L. P., and Blackburn, T. H.: Denitrification in Estuarine Sediment Stimulated by the Irrigation Activity of the Amphipod Corophium-Volutator, Mar. Ecol. Prog. Ser., 105, 285–290, https://doi.org/10.3354/meps105285, 1994.
Pinder, L.: The habitats of chironomid larvae, in: The Chironomidae, edited by: Armitage, P. D., Cranston, P. S., and Pinder, L. C. V., Springer, Dordrecht, 107–135, https://doi.org/10.1007/978-94-011-0715-0_6, 1995.
Plante, C. J., Jumars, P. A., and Baross, J. A.: Rapid bacterial growth in the hindgut of a marine deposit feeder, Microb. Ecol., 18, 29–44, 1989.
Premke, K., Karlsson, J., Steger, K., Gudasz, C., von Wachenfeldt, E., and Tranvik, L. J.: Stable isotope analysis of benthic fauna and their food sources in boreal lakes, J. N. Am. Benthol. Soc., 29, 1339–1348, https://doi.org/10.1899/10-002.1, 2010.
Raymond, P. A., Hartmann, J., Lauerwald, R., Sobek, S., McDonald, C., Hoover, M., Butman, D., Striegl, R., Mayorga, E., Humborg, C., Kortelainen, P., Durr, H., Meybeck, M., Ciais, P., and Guth, P.: Global carbon dioxide emissions from inland waters, Nature, 503, 355–359, https://doi.org/10.1038/nature12760, 2013.
Reuss, N. S., Hamerlik, L., Velle, G., Michelsen, A., Pedersen, O., and Brodersen, K. P.: Stable isotopes reveal that chironomids occupy several trophic levels within West Greenland lakes: Implications for food web studies, Limnol. Oceanogr., 58, 1023–1034, https://doi.org/10.4319/lo.2013.58.3.1023, 2013.
Robbins, J. A., Krezoski, J. R., and Mozley, S.: Radioactivity in sediments of the Great Lakes: post-depositional redistribution by deposit-feeding organisms, Earth Planet. Sc. Lett., 36, 325–333, 1977.
Roskosch, A., Hette, N., Hupfer, M., and Lewandowski, J.: Alteration of Chironomus plumosus ventilation activity and bioirrigation-mediated benthic fluxes by changes in temperature, oxygen concentration, and seasonal variations, Freshw. Sci., 31, 269–281, https://doi.org/10.1899/11-043.1, 2012.
Sabri, A., Leroy, P., Haubruge, E., Hance, T., Frere, I., Destain, J., and Thonart, P.: Isolation, pure culture and characterization of Serratia symbiotica sp. nov., the R-type of secondary endosymbiont of the black bean aphid Aphis fabae, Int. J. Syst. Evol. Micr., 61, 2081–2088, 2011.
Saether, O. A.: Chironomid communities as water quality indicators, Ecography, 2, 65–74, 1979.
Saether, O. A.: The influence of eutrophication on deep lake benthic invertebrate communities, Prog. Water Technol., 12, 161–180, https://doi.org/10.1016/B978-0-08-026024-2.50014-6, 1980.
Santos, H. A. and Massard, C. L.: The Family Holosporaceae, The Prokaryotes: Alphaproteobacteria and Betaproteobacteria, Springer, Berlin, Heidelberg, 237–246, 2014.
Scheffer, M., Hosper, S. H., Meijer, M. L., Moss, B., and Jeppesen, E.: Alternative Equilibria in Shallow Lakes, Trends Ecol. Evol., 8, 275–279, https://doi.org/10.1016/0169-5347(93)90254-M, 1993.
Schmieder, R. and Edwards, R. J. B.: Quality control and preprocessing of metagenomic datasets, Bioinformatics, 27, 863–864, 2011.
Sedlmeier, U. A. and Hoffmann, K. H.: Integumentary uptake of short-chain carboxylic acids by two freshwater oligochaetes, Tubifex tubifex and Lumbriculus variegatus: Specificity of uptake and characterization of transport carrier, J. Exp. Zool., 250, 128–134, 1989.
Senderovich, Y., Gershtein, Y., Halewa, E., and Halpern, M.: Vibrio cholerae and Aeromonas: do they share a mutual host?, ISME J., 2, 276–283, 2008.
Seuß, J., Hipp, E., and Hoffmann, K.: Oxygen consumption, glycogen content and the accumulation of metabolites in Tubifex during aerobic-anaerobic shift and under progressing anoxia, Comp. Biochem. Phys. A, 75, 557–562, 1983.
South, A.: Rworldmap: A New R package for Mapping Global Data, R J, 3, 35–43, 2011.
Stackebrandt, E., Lang, E., Cousin, S., Päuker, O., Brambilla, E., Kroppenstedt, R., and Lünsdorf, H.: Deefgea rivuli gen. nov., sp. nov., a member of the class Betaproteobacteria, Int. J. Syst. Evol. Micr., 57, 639–645, 2007.
Steinsberger, T., Schmid, M., Wüest, A., Schwefel, R., Wehrli, B., and Müller, B.: Organic carbon mass accumulation rate regulates the flux of reduced substances from the sediments of deep lakes, Biogeosciences, 14, 3275–3285, https://doi.org/10.5194/bg-14-3275-2017, 2017.
Stief, P., Nazarova, L., and de Beer, D.: Chimney construction by Chironomus riparius larvae in response to hypoxia: microbial implications for freshwater sediments, J. N. Am. Benthol. Soc., 24, 858–871, 2005.
Stief, P., Poulsen, M., Nielsen, L. P., Brix, H., and Schramm, A.: Nitrous oxide emission by aquatic macrofauna, P. Natl. Acad. Sci. USA, 106, 4296–4300, https://doi.org/10.1073/pnas.0808228106, 2009.
Stief, P.: Stimulation of microbial nitrogen cycling in aquatic ecosystems by benthic macrofauna: mechanisms and environmental implications, Biogeosciences, 10, 7829–7846, https://doi.org/10.5194/bg-10-7829-2013, 2013.
Svensson, J. M., Enrich-Prast, A., and Leonardson, L.: Nitrification and denitrification in a eutrophic lake sediment bioturbated by oligochaetes, Aquat. Microb. Ecol., 23, 177–186, 2001.
Tanner, A. and Paster, B. J.: The genus Wolinella, in: The Prokaryotes, Springer, New York, NY, 3512–3523, 1992.
Team, R. C.: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2012, http://www.R-project.org (last access: 20 February 2018), 2018.
Teixeira, L. and Merquior, V.: The family Moraxellaceae, in: The prokaryotes: Gammaproteobacteria, Springer, Berlin Heidelberg, 443–476, 2014.
Templeton, A. S., Chu, K. H., Alvarez-Cohen, L., and Conrad, M. E.: Variable carbon isotope fractionation expressed by aerobic CH4-oxidizing bacteria, Geochim. Cosmochim. Ac., 70, 1739–1752, https://doi.org/10.1016/j.gca.2005.12.002, 2006.
Timm, T.: Tubifex tubifex (Müller, 1774)(Oligochaeta, Tubificidae) in the profundal of Estonian lakes, Int. Rev. Ges. Hydrobio., 81, 589–596, 1996.
Verdonschot, P. F.: Macrofaunal community types in ponds and small lakes (Overijssel, the Netherlands), Hydrobiologia, 232, 111–132, 1992.
Vollenweider, R. and Kerekes, J.: Eutrophication of Waters: Monitoring, Assessment and Control. OECD, Paris, 154, ISBN 92-64-12298-2, 1982.
Wang, Y. and Qian, P.-Y.: Conservative Fragments in Bacterial 16S rRNA Genes and Primer Design for 16S Ribosomal DNA Amplicons in Metagenomic Studies, PLoS ONE, 4, e7401, https://doi.org/10.1371/journal.pone.0007401, 2009.
Wexler, H.: The genus Bacteroides, in: The Prokaryotes, edited by: Rosenberg, E., DeLong, E. F., Lory, S., Stackebrandt, E., and Thompson, F., Springer, Berlin, Heidelberg, https://doi.org/10.1007/978-3-642-38954-2_129, 2014.
White, D. S. and Miller, M. F.: Benthic invertebrate activity in lakes: linking present and historical bioturbation patterns, Aquat. Biol., 2, 269–277, https://doi.org/10.3354/ab00056, 2008.
Wrighton, K. C., Thomas, B. C., Sharon, I., Miller, C. S., Castelle, C. J., VerBerkmoes, N. C., Wilkins, M. J., Hettich, R. L., Lipton, M. S., and Williams, K. H.: Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla, Science, 337, 1661–1665, 2012.
Yasuno, N., Shikano, S., Shimada, T., Shindo, K., and Kikuchi, E.: Comparison of the exploitation of methane-derived carbon by tubicolous and non-tubicoloUs chironomid larvae in a temperate eutrophic lake, Limnology, 14, 239–246, 2013.





