the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Soil greenhouse gas fluxes from tropical coastal wetlands and alternative agricultural land uses
Emad Kavehei
Damien T. Maher
Stuart E. Bunn
Mehran Rezaei Rashti
Bahareh Shahrabi Farahani
Maria Fernanda Adame
Coastal wetlands are essential for regulating the global carbon budget through soil carbon sequestration and greenhouse gas (GHG – CO2, CH4, and N2O) fluxes. The conversion of coastal wetlands to agricultural land alters these fluxes' magnitude and direction (uptake/release). However, the extent and drivers of change of GHG fluxes are still unknown for many tropical regions. We measured soil GHG fluxes from three natural coastal wetlands – mangroves, salt marsh, and freshwater tidal forests – and two alternative agricultural land uses – sugarcane farming and pastures for cattle grazing (ponded and dry conditions). We assessed variations throughout different climatic conditions (dry–cool, dry–hot, and wet–hot) within 2 years of measurements (2018–2020) in tropical Australia. The wet pasture had by far the highest CH4 emissions with 1231±386 , which were 200-fold higher than any other site. Dry pastures and sugarcane were the highest emitters of N2O with 55±9 (wet–hot period) and 11±3 m (hot-dry period, coinciding with fertilisation), respectively. Dry pastures were also the highest emitters of CO2 with 20±1 (wet–hot period). The three coastal wetlands measured had lower emissions, with salt marsh uptake of and of N2O and CO2, respectively, during the dry–hot period. During the sampled period, sugarcane and pastures had higher total cumulative soil GHG emissions (CH4+N2O) of 7142 and 56 124 compared to coastal wetlands with 144 to 884 (where CO2-eq is CO2 equivalent). Restoring unproductive sugarcane land or pastures (especially ponded ones) to coastal wetlands could provide significant GHG mitigation.
- Article
(2086 KB) - Full-text XML
-
Supplement
(1101 KB) - BibTeX
- EndNote
Coastal wetlands are found at the interface of terrestrial and marine ecosystems and account for 10 % of the global wetland area (Lehner and Döll, 2004). They are highly productive and provide various ecosystem services such as water quality improvement, biodiversity, and carbon sequestration (Duarte et al., 2013). For instance, mangroves can accumulate 5 times more soil carbon than terrestrial forests (Kauffman et al., 2020). However, the high productivity and anoxic soil conditions that promote carbon sequestration can also favour potent greenhouse gas (GHG) emissions, including CO2, CH4, and N2O (Whalen, 2005; Conrad, 2009).
The GHG emissions in coastal wetlands primarily result from microbial processes in the soil–water–atmosphere interface (Bauza et al., 2002; Whalen, 2005). The emission of CO2 is a result of respiration, where fixed carbon by photosynthesis is partially released back into the atmosphere (Oertel et al., 2016). Emissions of CH4 result from anaerobic and aerobic respiration by methanogenic bacteria, mostly in waterlogged conditions (Angle et al., 2017; Saunois et al., 2020). Finally, N2O emissions are caused by denitrification in anoxic conditions and nitrification in aerobic soils, both driven by nitrogen content and soil moisture (Ussiri and Lal, 2012). Thus, the total GHG emissions from a wetland are driven by environmental conditions that favour these microbial processes, all of which result in highly variable emissions from wetlands worldwide (Kirschke et al., 2013; Oertel et al., 2016).
Despite potentially high GHG emissions from coastal wetlands, these are likely to be lower than those from alternative agricultural land use (Knox et al., 2015), which emit GHGs from their construction and throughout their productive lives. Firstly, when wetlands are converted to agricultural land, the oxidation of sequestered carbon in the organic-rich soils releases significant amounts of CO2 (Drexler et al., 2009; Hooijer et al., 2012). Secondly, removing tidal flow and converting coastal wetlands to freshwater systems, such as during the creation of ponded pastures, dams, or agricultural ditches, can result in very high CH4 emissions (Deemer et al., 2016; Grinham et al., 2018; Ollivier et al., 2019). For instance, agricultural ditches contribute up to 3 % of the total anthropogenic CH4 emissions globally (Peacock et al., 2021). Finally, the use of fertilisers significantly increases N2O emissions (Rezaei Rashti et al., 2015). Thus, emissions of GHGs from land use change can be mitigated through the reversal of these activities, for instance, reduction in fertiliser use and the reinstallation of tidal flow on unused agricultural land (Kroeger et al., 2017; Rezaei Rashti et al., 2015).
This study measured the annual GHG fluxes (CO2, CH4, and N2O) from three natural coastal wetlands (mangroves, salt marsh and freshwater tidal forests) and two agricultural land use sites (sugarcane plantation and pasture) in tropical Australia. The objectives were to assess the difference in GHG fluxes throughout different seasons that characterise tropical climates (dry–cool, dry–hot, and wet–hot) and identify environmental factors associated with these GHG fluxes. These data will inform emission factors for converting wetlands to agricultural land uses and vice versa, filling in a knowledge gap identified in Australia (Baldock et al., 2012) and tropical regions worldwide (IPCC, 2013).
2.1 Study sites
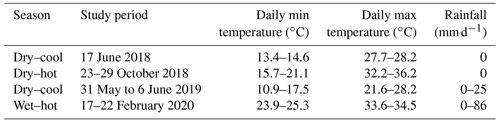
The study area is located within the Herbert River catchment in Queensland, northeast Australia (Fig. 1a). The region has a tropical climate with a mean monthly minimum temperature from 14 to 23 ∘C and mean monthly maximum temperature from 25 to 33 ∘C (Australian Bureau of Meteorology, ABM, 2020; 1968–2020; Sect. S4 in the Supplement). The average rainfall is 2158 mm yr−1, with the highest values of 476 mm during February (ABM, 2020; 1968–2020; Sect. S4). The Herbert basin covers 9842 km2, from which 56 % is grazing, 31 % is conserved wetlands and forestry, 8 % is sugarcane, and 4 % is other land uses (Department of Environment and Science, Queensland, 2013). Wetlands in this region were heavily deforested in the last century (1943–1996) due to rapid agricultural development, primarily for sugarcane farming (Griggs, 2018). Before clearing, the land was mostly covered by rainforest and coastal wetlands, mainly Melaleuca forest, grass, and sedge swamps (Johnson et al., 1999).
We selected five sites, including three natural coastal wetlands (Fig. 1) – a mangrove forest ( S, E), a salt marsh ( S, E), and a freshwater tidal forest ( S, E) – and two common agricultural land use types of the region – a sugarcane crop ( S, E) and a pasture for fodder grazing. The pasture had different levels of inundation: some areas were covered with shallow ponds (50–100 cm depth); some were wet (hereafter “wet pasture”; S, E); and others were dry (hereafter, “dry pasture”; S, E). The natural coastal wetlands and the sugarcane site were located within the same property <200 m apart at Insulator Creek (Fig. 1b), while the ponded pasture was 20 km north at Mungalla Station (Fig. 1a). The mangroves were dominated by Avicennia marina with a few plants of Rhizophora stylosa, and the salt marsh was dominated by Suaeda salsa and Sporobolus spp. Landwards, the freshwater tidal forest, a wetland commonly known as “tea tree swamp”, was dominated by Melaleuca quinquenervia trees. While the mangroves and salt marsh are directly submerged by tides (5–30 cm), the freshwater tidal forest is indirectly affected by tidal fluctuations, such as during large spring tides, when tidal water can push groundwater above the forest thus forming “supra-tidal” wetlands. The coastal wetlands were adjacent to a sugarcane farm of ∼110 ha (Fig. 1b). The sugarcane is fertilised once a year with urea at a rate of 135 kg N ha−1 and harvested during May–June, and the foliage is left on the soil surface (trash blanket) after harvest. The ponded pastures in Mungalla Station comprise a 250 ha farm and support ∼900 cattle by providing fodder during dry periods. The selected ponded pastures were covered by Eichhornia crassipes (water hyacinth) and Hymenachne amplexicaulis (Fig. 1g–h). Each of the five sites was sampled during three periods: dry–cool (May–September), dry–hot (October–December), and wet–hot (January–April; Table 1). During each time period, soil physicochemical properties and GHG fluxes were measured as detailed below.
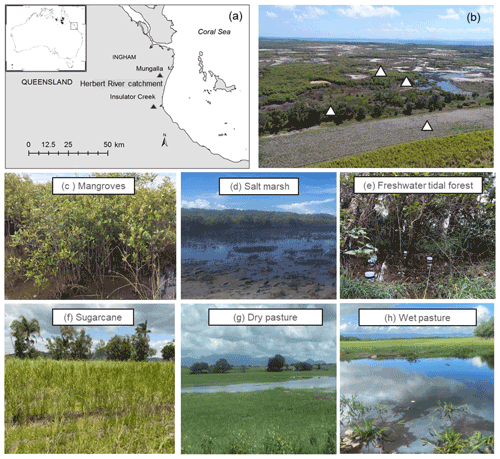
Figure 1(a) Location of sampling sites (Insulator Creek and Mungalla) in the Herbert River catchment, northeast Australia; (b) natural wetlands adjacent to sugarcane in Insulator Creek and sampling locations; and (c) mangroves, (d) salt marsh, (e) freshwater tidal forest, (f) sugarcane, (g) dry pasture, and (h) wet pasture. Pictures by Naima Iram and Maria Fernanda Adame.
2.2 Soil sampling and analysis of physicochemical properties
Soil physicochemical characteristics were measured in composite soil samples next to each gas chamber location (n=5; 0–30 cm) for all study sites during the dry–hot season. The samples were obtained by inserting an open steel corer to a depth of 30 cm; the core was divided into three depths: 0–10, 10–20, and 20–30 cm. Soil samples were oven-dried at 105 ∘C for 48 h to determine volumetric water content through gravimetric analysis. The volumetric water content was divided by total soil porosity to determine water-filled pore space (WFPS). Total soil porosity was calculated using an equation from Rezaei Rashti et al. (2015; Eq. 1):
Soil texture analysis (% sand, silt, clay) was performed with a simplified method for particle size determination (Kettler et al., 2001). Soil electrical conductivity (EC) and pH were measured using a conductivity meter (TPS WP-84, Australia) in soil–water slurry at 1:5. Soil subsamples were air-dried, sieved (2 mm), ground (Retsch™ mill), and analysed for the percentage of N (%N) and percentage of C (%C) using an elemental analyser connected to a gas isotope ratio mass spectrometer (EA–Delta V Advantage IRMS, Griffith University). Additionally, soil samples from the top 10 cm were collected during each sampling to measure gravimetric soil moisture content and bulk density.
2.3 Greenhouse gas fluxes
We measured GHG fluxes (CO2, CH4, and N2O) at each site for 3 consecutive days during each sampling period except for the dry–cool period of 2018, when mangroves, salt marsh, and sugarcane were surveyed for 1 d. The sampling was carried out between 09:00 and 11:00 LT, representing the mean daily temperatures, thus minimising variability in cumulative seasonal fluxes based on intermittent manual flux measurements (Reeves et al., 2016). Additionally, we assessed the variability in our measurements with tidal inundation in mangroves and salt marsh, which were regularly inundated (∼10–30 cm) during the hot-dry season. However, due to logistic constraints, further sampling was conducted only during low tides.
We used static, manual gas chambers made of high-density, round polyvinyl chloride pipe, which consisted of two units: a base (r=12 cm; h=18 cm) and a detachable collar (h=12 cm; Hutchinson and Mosier, 1981; Kavehei et al., 2021). The chambers had lateral holes that could be left covered with rubber bungs at low water levels and left open at high water levels to allow water movement between sampling events. When the wetlands were inundated for the experiments, we used PVC extensions (h=18 cm). Five chambers were set ∼5 cm deep in the soil; separated 1 to 2 m from each other; and selectively located on soil with minimal vegetation, roots, and crab burrows. The chambers were set 1 d before sampling to minimise installation disturbance during the experiment (Rezaei Rashti et al., 2015). We were careful not to tramp around the chambers during installation and sampling. The fact that emissions were not significantly different among days (p>0.05) provided us with confidence that disturbance due to installation was not problematic.
At the start of the experiment, gas chambers were closed. A sample was taken at time zero and then after 1 h with a 20 mL syringe and transferred to a 12 mL vacuumed container (Exetainer, Labco Ltd, High Wycombe, UK). During the dry–hot season, linearity tests of GHG fluxes with time were conducted by sampling at 0, 20, 40, and 60 min at all chambers (Rezaei Rashti et al., 2016). For the rest of the experiments, linearity tests were performed in one chamber per site; R2 values were consistently above 0.70. During each experiment, soil temperature was measured next to each chamber. For each experiment, the base depth was recorded from five points within each chamber to calculate headspace volume. The obtained volumetric unit concentrations were converted to mass-based units using the ideal gas law (Hutchinson and Mosier, 1981).
The GHG concentrations of all samples were analysed within 2 weeks of sampling with a gas chromatograph (Shimadzu GC-2010 Plus). For N2O analysis, an electron capture detector was used with helium as the carrier gas, while CH4 was analysed on a flame ionisation detector with nitrogen as the carrier gas. For CO2 determination, the gas chromatograph was equipped with a thermal conductivity detector. Peak areas of the samples were compared against standard curves to determine concentrations (Chen et al., 2012). Seasonal cumulative GHG fluxes were calculated by modifying the equation described by Shaaban et al. (2015; Eq. 2):
where Ri is the gas emission rate ( for CO2 and for CH4 and N2O); Di is the mean daily GHG emission rate in a season ( for CO2 and for CH4 and N2O) during low tide; and 17.38 is the number of weeks in each season, assuming these conditions were representative of the annual cycle (see Table 1).
Annual cumulative soil GHG fluxes (CH4+N2O) were calculated by integrating cumulative seasonal fluxes. These estimations did not account for soil CO2 values as our methodology with dark chambers only accounted for emissions from respiration and excluded uptake by primary productivity. The CO2-equivalent (CO2-eq) values were estimated by multiplying CH4 and N2O emissions by 25 and 298, respectively (Solomon, 2007), which represented the radiative balance of these gases (Neubauer, 2021). For annual cumulative soil GHG flux calculations from coastal wetlands, we used GHG fluxes measured during low tide; therefore, our values did not incorporate the effect of tidal fluctuations. The spatial and temporal replication of this study targeted spatial variation within soil type (<50 cm, five chambers), days (3 d per sampling), and seasons (three seasons per year). However, our replication within land use and wetland type was limited; thus, generalisations for all wetlands and land uses should be made acknowledging this limitation.
2.4 Statistical analyses
GHG flux data were tested for normality through Kolmogorov–Smirnov and Shapiro–Wilk tests. The data were then analysed for spatial and temporal differences with one-way analyses of variance (ANOVAs), where site and season were the predictive factors and the replicate (chamber) was the random factor of the model. When data were not normal, they were transformed (log10 or ) to comply with the assumptions of normality and homogeneity of variances. Some variables were not normally distributed despite transformations and were analysed with the non-parametric Kruskal–Wallis test and Mann–Whitney U test. A Pearson correlation test was run to evaluate the correlation of GHGs with measured environmental factors. Analyses were performed with SPSS (v25, IBM, New York, USA), and values are presented as the mean ± standard error (SE).
3.1 Soil physicochemical properties
Soil physical and chemical parameters (mean values 0–30 cm) varied among sites (Table 2; see full results of statistical analyses in Sect. S5 in the Supplement). As expected, gravimetric moisture content was highest in the coastal wetlands and wet pasture (>26 %) and lowest in the sugarcane and the dry pasture (<14 %). All soils were acidic, especially the freshwater tidal forest and the wet pastures with values <5 throughout the sediment column; mangroves had the highest pH with 6.0±0.1. The lowest EC was recorded in the pastures (247±38 and 190±39 µS cm−1 for the dry and wet pasture, respectively) and highest in the three natural coastal wetlands with 1418±104, 8049±276, and 8930±790 µS cm−1 for the freshwater tidal forest, salt marsh, and mangroves, respectively.
Soil bulk density was highest in sugarcane (1.5±0.1 g cm−3) and lowest in the freshwater tidal forest (0.6±0.1 g cm−3). For all sites, %C was highest in the top 10 cm of the soil and decreased with depth, with the highest values in the freshwater tidal forest (5.1±0.6 %) and lowest in the salt marsh (1.2±0.1 %). Soil %N ranged from 0.1±0.0 % to 0.4±0.1 % in all sites, except in the freshwater tidal wetland, where it reached values of 0.6±0.0 % in the top 10 cm (Table 2).
3.2 Greenhouse gas fluxes
Soil emissions for CO2 were significantly different among sites and times of the year (t=155.09 and n=237 with p<0.001; Fig. 2a). The highest CO2 emissions were measured during the wet–hot period in the dry pasture, where values reached 20 308±1951 , while the lowest values were measured in the salt marsh, the only site that acted as a sink of CO2 with an uptake rate of . In the pastures, CO2 emissions were twice as high when dry, with cumulative annual emissions of 5748±303 , compared to when wet, with 2163±465 . For the coastal wetlands, cumulative annual CO2 emissions were highest in freshwater tidal forests with 2213±284 , followed by mangroves with 1493±111 , and lowest at the salt marsh with uptake rates of .
For CH4 fluxes, significant differences were observed among sites and seasons (t=182.33 and n=237 with p<0.001). The differences between different sites were substantial, with wet pasture having significantly higher CH4 emissions than any other site at rates ∼200 times higher (Fig. 2b). For tidal coastal wetlands, emissions of CH4 were highest during the wet–hot season in all the sites except for the mangroves, which had similar emissions throughout the year (Fig. 2b). Overall, cumulative annual CH4 emissions were 209±36 for the wet pasture followed by mangroves (0.73±0.13 ), dry pasture (0.15±0.03 ), freshwater tidal forest (0.14±0.03 ), salt marsh (0.04±0.01 ), and sugarcane ( ).
For N2O fluxes, the highest emissions (54.6±9.0 ) were from the dry pasture in the wet–hot season, followed by sugarcane (20.5±2.7 ) during the hot-dry period, which coincides with the post-fertilisation months (Fig. 2c). Overall, dry pastures had the highest cumulative annual N2O emissions (7.99±2.26 ), followed by sugarcane (2.37±0.68 ), wet pasture (1.32±0.33 ), salt marsh (0.33±0.11 ), freshwater tidal forests (0.04±0.0 ), and finally mangroves (0.02±0.04 ). However, these differences were only significant when considering the interaction between the time of the year and site (t=100.21 and n=237 with p<0.001).
The CH4 fluxes did not vary significantly between the low and high tide within all coastal wetlands. Contrarily, for salt marsh, CO2 was taken during the high tide ( ) but emitted (0.69±0.4 ) during the low tide (F1,28=20.06 with p<0.001). Finally, for N2O, fluxes differed in all coastal wetlands, with higher uptakes in the high tide for mangroves (F1,28=38.28 with p<0.001; F1,28=13.53 with p=0.001) and higher emissions in salt marsh (F1,28=38.31 with p<0.001) during low tide (Sect. S3). These results suggested that there was a likely variability for CO2 and N2O fluxes, depending on the time of sampling.
The wet pasture had the highest total cumulative soil GHG emissions (CH4+N2O) with 56 124 CO2-eq followed by dry pasture at 23 890 CO2-eq and sugarcane at 7142 CO2-eq , while coastal wetlands had comparatively lower cumulative soil GHG emissions with 884, 235, and 144 CO2-eq for salt marsh, mangroves, and freshwater tidal forests, respectively. Overall, the three coastal wetlands measured in this study had lower total cumulative GHG emissions at 1263 compared to the alternate agricultural land uses, which emitted 87 156 .
3.3 Greenhouse gas emissions and environmental factors
Overall, we found that not one single parameter measured in this study could explain GHG fluxes for all sites except land use. The CO2 emissions were not significantly correlated with bulk density (R2=0.026; p=0.918; n=18), percent WFPS (% WFPS) (; p=0.99; n=18), or soil temperature (R2=0.296; p=0.233; n=18). Soil CH4 emissions were not correlated with bulk density (; p=0.706; n=18), % WFPS (R2=0.224; p=0.372; n=18), or soil temperature (R2=0.286; p=0.25; n=18). Finally, no correlation was found between N2O emissions and bulk density (; p=0.156; n=18), % WFPS (; p=0.168; n=18), or soil temperature (; p=0.335; n=18). The full raw dataset of GHG fluxes is provided in Sect. S1.
Table 2Physicochemical characteristics for the soil of natural coastal wetlands, sugarcane, and pastures (dry and ponded) for the top 30 cm of soil in tropical Australia. C is carbon; N is nitrogen; EC is electrical conductivity. Values are the mean ± standard error (n=5).
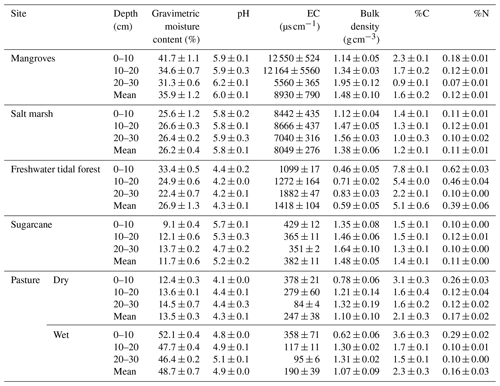
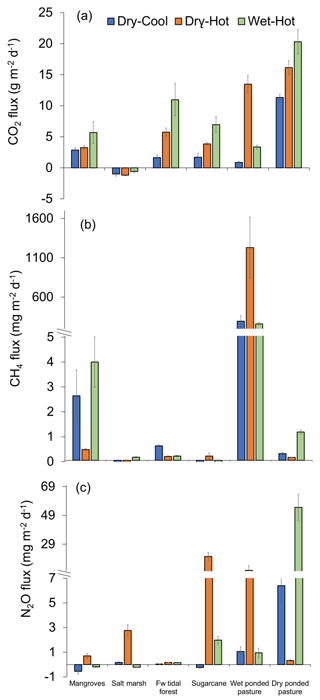
Figure 2Greenhouse gas fluxes () of (a) CO2, (b) CH4, and (c) N2O from the soils of tropical coastal wetlands – mangroves, salt marsh, freshwater (Fw) tidal forest – and two alternative land uses – sugarcane and pastures (wet and dry) – during three periods of the year: dry–cool, dry–hot, and wet–hot.
The soils of the three coastal wetlands measured in this study (mangroves, salt marshes, and freshwater tidal forests) had significantly lower GHG emissions than those from two alternative land uses common in tropical Australia (sugarcane and grazing pastures). Notably, we found that coastal wetlands had 200-times-lower CH4 emissions and 7-times-lower N2O compared to wet pastures and sugarcane soils, respectively. These results support our hypothesis that the management or conversion of unused sugarcane land and ponded pastures in tropical Australia could be restored to coastal wetlands and result in significant GHG mitigation.
The variability in GHG fluxes was best explained by land use and wetland type; however, some trends with seasons were evident. For instance, CO2 and N2O emissions were lowest during the dry–cool periods. Reduced emissions at low temperatures are expected as the temperature is a primary driver of any metabolic process, including respiration and nitrification–denitrification. Mangroves tend to have higher CO2 emissions as temperature increases (Liu and Lai, 2019), and forests have significantly higher N2O emissions during warm seasons (Schindlbacher et al., 2004). Emissions of CH4 also tend to increase with temperature as the activity of methane-producing soil microbes (Ding et al., 2004) and the availability of carbon are higher in warmer conditions (Yvon-Durocher et al., 2011). However, most studies to date on GHG fluxes have been conducted in temperate locations, where temperature differences throughout the year are smaller than in tropical regions. For tropical regions, increased GHG emissions are likely to be strongly affected by the “Birch effect”, which refers to a short-term but substantial increase in respiration from soils under the effect of precipitation during the early wet season (Fernandez-Bou et al., 2020).
The main factor associated with GHG fluxes was land use and type of wetland. Notably, coastal wetlands, even the freshwater tidal forests, had much lower emissions than wet pastures. This significant difference could be attributed to terminal electron acceptors in the soils (e.g. iron, sulfate, manganese) of the coastal wetlands, which could inhibit methanogenesis (Kögel-Knabner et al., 2010; Sahrawat, 2004). Sulfate-reducing bacteria are also likely to outcompete methane-producing bacteria (methanogens) in the presence of high sulfate concentrations in tidal wetlands, resulting in low CH4 production. Competition between methanogens and methanotrophs may result in a net balance of low CH4 production despite freshwater conditions (Maietta et al., 2020). Additionally, microorganisms living within the bark of Melaleuca trees can consume CH4 (Jeffrey et al., 2021), so it is possible that similar bacteria could reduce CH4 emissions in the soil. Interestingly, variability within CH4 fluxes among sites was very high, despite being very close to each other (Fig. 1b). These differences highlight the importance of land use in GHG fluxes, which are likely to significantly alter the microbial community composition and abundance, changing rapidly over small spatial scales (Drenovsky et al., 2010; Martiny et al., 2006).
Our results are consistent with other studies, showing the importance of land use in GHG emissions. For instance, in a Mediterranean climate, the drained agricultural land use types, pasture and corn, were larger CO2 emitters than restored wetlands (Knox et al., 2015). Clearing of wetlands for agricultural development, such as the drainage of peatlands, results in very high CO2 emissions (Hirano et al., 2012; Nieveen et al., 2005; Veenendaal et al., 2007), and restoration of these wetlands could decrease these emissions (Cameron et al., 2021). Additionally, some of the wetland types, such as marshes, were occasional sinks of CO2 and CH4, consistent with previous studies where intertidal wetlands are a sink of GHGs at least under some conditions or during some times of the year (Knox et al., 2015; Maher et al., 2016).
The fluxes measured in the coastal wetlands of this study, −1191 to 10 970 for CO2, −0.3 to 3.9 for CH4, and −0.2 to 2.8 for N2O, were within the range of those measured in other subtropical/tropical wetlands worldwide (except for the negative CO2 fluxes in salt marsh soils, Table 3). Fluxes can range from 44 to 11 328 for CO2, from 0.03 to 1255 for CH4, and from 0.1 to 279 for N2O (Table 3). Despite being in tropical regions, GHG fluxes from this study were lower compared to other climates (Table 3). Contrary to previous studies, CO2 uptake by salt marsh soil was likely to be linked with dark CO2 fixation in wetland soils (Akinyede et al., 2020; Mar Lynn et al., 2017). Wetland soils exhibit autotrophic bacteria which contribute to dark CO2 fixation at ∼311 ; however these rates could vary depending upon abundance and diversity of microbial communities (Akinyede et al., 2020). Further studies exploring the presence and abundance of CO2-fixing bacteria in salt marsh soils are recommended. The general lower nitrogen pollution in Australia's soils and waterways compared to other countries may partially explain the lower emissions. However, the GHG flux measurements from this study did not account for the effects of vegetation, which can alter fluxes. For instance, some plant species of rice paddies (Timilsina et al., 2020) and Miscanthus sinensis (Lenhart et al., 2019) can increase N2O emissions, and some tree species can facilitate CH4 efflux from the soil (Pangala et al., 2013). Finally, changes in emissions between low and high tides were detected for CO2 and N2O. Thus, future studies that include vegetation and changes within tidal cycles will improve GHG flux estimates for coastal wetlands.
Table 3Comparison of GHG fluxes () with other studies.
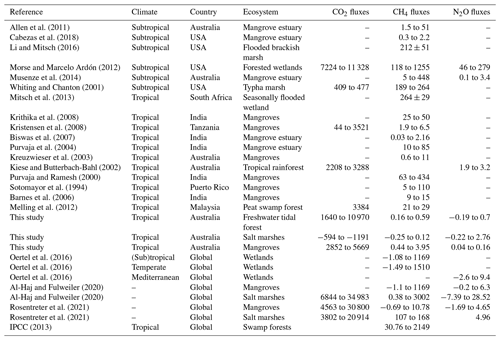
Note that a dash means no data were available; GHG fluxes as CO2–C, CH4–C, and N2O–N were multiplied by 3.66, 1.34, and 1.57, respectively, to calculate CO2, CH4, and N2O fluxes (National Greenhouse Accounts Factors, Australian Government Department of Industry, Science, Energy and Resources, 2020).
4.1 Management implications
Under the Paris Agreement, Australia has committed to reducing GHG emissions to 26 %–28 % below its 2005 levels by 2030. With annual emissions of 153×106 t of carbon dioxide equivalent (Mt CO2-eq yr−1), Queensland is a major GHG emitter in Australia (∼28.7 % of the total in 2016; Department of Environment and Science, Queensland, 2016). Of these emissions, about 18.3 Mt CO2-eq yr−1 (14 %) are attributed to agriculture, while land use change and forestry emit 12.1 Mt CO2-eq yr−1 (DES, 2016). Production of CH4 from ruminant animals, primarily cattle, contribute 82 % of agriculture-related emissions (DES, 2016). Therefore, any GHG mitigation strategy involving land use change could be important for Australia to achieve its national goals.
This study supports the application of three management actions that could reduce GHG emissions. First, the conversion of ponded pastures to coastal wetlands is likely to reduce soil GHG emissions. Our results showed that wet pastures emit 56 of the total GHG emissions (CH4+N2O) compared with 0.2 , 0.1 , and 0.9 from mangroves, freshwater tidal forest, and salt marshes, respectively. This implies that about 55 of emissions from the soils could be potentially avoided by converting wet pastures to coastal wetlands. The carbon mitigation for GHG emissions only from soil could provide ∼AUD 894 , assuming a carbon value of AUD 15.99 per tonne of CO2-eq (Australian Government Clean Energy Regulator, 2021). This mitigation could be added to the carbon sequestration through sediment accumulation and tree growth that results from wetland restoration. Legal enablers in Queensland are in place to manage unproductive agricultural land this way (Bell-James and Lovelock, 2019) and could provide an alternative income source for farmers.
A second management option would be to reduce the time pastures are kept underwater. Dry pastures produced significantly less CH4 with ∼0.005 than wet pastures with 6 . For comparison, an average cow produces 141 g CH4 d−1 (McGinn et al., 2011), and our study farm supported around 900 cattle over 250 ha throughout the year, equivalent to 185 compared to 2 and 2090 of CH4 from dry and wet pasture, respectively. This implies that nearly 92 % of the CH4 emissions came from wet pastures, while dry pasture and grazing cattle had a low share in total CH4 emissions in this case scenario. Therefore, land use management of wet pastures may be an opportunity to reduce agriculture-related CH4 emissions. Future studies should increase the number of sites of ponded pastures to account for variability in hydrology, fertilisation, and on-farm cattle density. However, the exceptionally large difference (2–3 orders of magnitude) between dry and ponded pastures and other coastal wetlands provides confidence that pasture management could provide significant GHG mitigation.
Finally, fertiliser management in sugarcane could reduce N2O emissions. Higher N2O emissions of 17.6 were measured in the sugarcane crop following fertilisation during the dry–hot season. Comparatively, natural wetlands had low N2O emissions (0.16 to 2.79 ), and even the salt marsh was an occasional sink. Thus, improved management of fertiliser applications could result in GHG emission mitigation. Some activities include the split application of nitrogen fertiliser in combination with low irrigation, reduction in fertiliser application rates, the substitution of nitrate-based fertiliser for urea (Rezaei Rashti et al., 2015), removing the mulch layer before fertiliser application (Pinheiro et al., 2019; Xu et al., 2019; Zaehle and Dalmonech, 2011), or the conversion of unproductive sugarcane to coastal wetlands.
The GHG emissions from three coastal wetlands in tropical Australia (mangroves, salt marsh, and freshwater tidal forests) were consistently lower than those from two common agricultural land uses of the region (sugarcane and pastures) across three climatic conditions (dry–cool, dry–hot, and wet–hot). Ponded pastures emitted 200 times more CH4 and sugarcane emitted 7 times more N2O than any natural coastal wetland measured. If these high emissions are persistent in other locations and within other tropical regions, conversion of pastures and sugarcane to similar coastal wetlands could provide significant GHG mitigation. As nations try to reach their emission reduction targets, projects aimed at converting or restoring coastal wetlands can financially benefit farmers while providing additional co-benefits.
All data are provided in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-18-5085-2021-supplement.
NI and MFA designed the project. NI, BSF, and EK carried out experiments. NI, EK, and MFA analysed the data. NI and MFA prepared the manuscript with contributions from DTM, SEB, and MRR.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We acknowledge the traditional owners of the land in which the field study was conducted, especially the Nywaigi people from Mungalla Station. We are also thankful to Sam and Santo Lamari for allowing us to work on their property and for sharing their local knowledge. We are thankful to Charles Cadier and Julieta Gamboa for their contribution to the fieldwork. We are very thankful to the anonymous referees for their valuable feedback, which helped to improve the quality of the article.
This research has been supported by the QLD Government (Industry Research Fellowship – Advance Queensland).
This paper was edited by Sara Vicca and reviewed by two anonymous referees.
Al-Haj, A. N. and Fulweiler, R. W.: A synthesis of methane emissions from shallow vegetated coastal ecosystems, Glob. Change Biol., 26, 2988–3005, https://doi.org/10.1111/gcb.15046, 2020.
Akinyede, R., Taubert, M., Schrumpf, M., Trumbore, S., and Küsel, K.: Rates of dark CO2 fixation are driven by microbial biomass in a temperate forest soil, Soil Biol. Biochem., 150, 107950, https://doi.org/10.1016/J.SOILBIO.2020.107950, 2020.
Allen, D., Dalal, R. C., Rennenberg, H., and Schmidt, S.: Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia, Plant Biol., 13, 126–133, https://doi.org/10.1111/j.1438-8677.2010.00331.x, 2011.
Angle, J. C., Morin, T. H., Solden, L. M., Narrowe, A. B., Smith, G. J., Borton, M. A., Rey-Sanchez, C., Daly, R. A., Mirfenderesgi, G., Hoyt, D. W., Riley, W. J., Miller, C. S., Bohrer, G., and Wrighton, K. C.: Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions, Nat. Commun., 8, 1567, https://doi.org/10.1038/s41467-017-01753-4, 2017.
Australian Bureau of Meteorology, ABM: Monthly Climate Statistics for “LUCINDA POINT” [032141], available at: http://www.bom.gov.au/jsp/ncc/cdio/cvg/av (last access: 5 May 2021), 2020.
Australian Government Clean Energy Regulator: Quarterly Carbon Market report, March 2021, available at: http://www.cleanenergyregulator.gov.au/DocumentAssets/Documents/Quarterly%20Carbon%20Market%20Report%20-%20March%20Quarter%202021.pdf , last access: 11 August 2021.
Baldock, J. A., Wheeler, I., McKenzie, N., and McBrateny, A.:, Crop Pasture Sci., 63, 269–283, https://doi.org/10.1071/CP11170, 2012.
Barnes, J., Ramesh, R., Purvaja, R., Rajkumar, A. N., Kumar, B. S., Krithika, K., Ravichandran, K., Uher, G., and Upstill-Goddard, R.: Tidal dynamics and rainfall control N2O and CH4 emissions from a pristine mangrove creek, Geophys. Res. Lett., 33, L15405, https://doi.org/10.1029/2006GL026829, 2006.
Bauza, J. F., Morell, J. M., and Corredor, J. E.: Biogeochemistry of nitrous oxide production in the red mangrove (Rhizophora mangle) forest sediments, Estuar. Coast. Shelf S., 55, 697–704, https://doi.org/10.1006/ecss.2001.0913, 2002.
Bell-James, J. and Lovelock, C. E.: Legal barriers and enablers for reintroducing tides: An Australian case study in reconverting ponded pasture for climate change mitigation, Land use policy, 88, 104192, https://doi.org/10.1016/J.LANDUSEPOL.2019.104192, 2019.
Biswas, H., Mukhopadhyay, S. K., Sen, S., and Jana, T. K.: Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India, J. Marine Syst., 68, 55–64, https://doi.org/10.1016/j.jmarsys.2006.11.001, 2007.
Cabezas, A., Mitsch, W. J., MacDonnell, C., Zhang, L., Bydałek, F., and Lasso, A.: Methane emissions from mangrove soils in hydrologically disturbed and reference mangrove tidal creeks in southwest Florida, Ecol. Eng., 114, 57–65, https://doi.org/10.1016/j.ecoleng.2017.08.041, 2018.
Cameron, C., Hutley, L. B., Munksgaard, N. C., Phan, S., Aung, T., Thinn, T., Aye, W. M., and Lovelock, C. E.: Impact of an extreme monsoon on CO2 and CH4 fluxes from mangrove soils of the Ayeyarwady Delta, Myanmar, Sci. Total Environ., 760, 143422, https://doi.org/10.1016/J.SCITOTENV.2020.143422, 2021.
Chen, G. C., Tam, N. F. Y., and Ye, Y.: Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics, Soil Biol. Biochem., 48, 175–181, https://doi.org/10.1016/j.soilbio.2012.01.029, 2012.
Conrad, R.: The global methane cycle: recent advances in understanding the microbial processes involved, Env. Microbiol. Rep., 1, 285–292, https://doi.org/10.1111/j.1758-2229.2009.00038.x, 2009.
Deemer, B. R., Harrison, J. A., Li, S., Beaulieu, J. J., Delsontro, T., Barros, N., Bezerra-Neto, J. F., Powers, S. M., Dos Santos, M. A., and Vonk, J. A.: Greenhouse Gas Emissions from Reservoir Water Surfaces: A New Global Synthesis, Bioscience, 66, 949–964, https://doi.org/10.1093/biosci/biw117, 2016.
Department of Environment and Science, Queensland: Herbert drainage basin – facts and maps, WetlandInfo website, available at: https://wetlandinfo.des.qld.gov.au/wetlands/facts-maps/basin-herbert/ (last access: 13 January 2021), 2013.
Department of Environment and Science, Queensland: State of the environment report, Total greenhouse gas emissions, available at: https://www.stateoftheenvironment.des.qld.gov.au/pollution/greenhouse-gas-emissions/total-annual-greenhouse-gas-emissions (last access: 5 April 2021), 2016.
Ding, W. X., Cai, Z. C., and Tsuruta, H.: Cultivation, nitrogen fertilization, and set-aside effects on methane uptake in a drained marsh soil in Northeast China, Glob. Change Biol., 10, 1801–1809, https://doi.org/10.1111/j.1365-2486.2004.00843.x, 2004.
Drenovsky, R. E., Steenwerth, K. L., Jackson, L. E., and Scow, K. M.: Land use and climatic factors structure regional patterns in soil microbial communities, Global Ecol. Biogeogr., 19, 27–39, https://doi.org/10.1111/j.1466-8238.2009.00486.x, 2010.
Drexler, J. Z., de Fontaine, C. S., and Deverel, S. J.: The legacy of wetland drainage on the remaining peat in the Sacramento–San Joaquin Delta, California, USA, Wetlands, 29, 372–386, 2009.
Duarte, C. M., Losada, I. J., Hendriks, I. E., Mazarrasa, I., and Marbà, N.: The role of coastal plant communities for climate change mitigation and adaptation, Nat. Clim. Chang., 3, 961–968, https://doi.org/10.1038/nclimate1970, 2013.
Fernandez-Bou, A. S., Dierick, D., Allen, M. F., and Harmon, T. C.: Precipitation-drainage cycles lead to hot moments in soil carbon dioxide dynamics in a Neotropical wet forest, Glob. Change Biol., 26, 5303–5319, https://doi.org/10.1111/gcb.15194, 2020.
Griggs, P. D.: Too much water: drainage schemes and landscape change in the sugar-producing areas of Queensland, 1920–90, Aust. Geogr., 49, 81–105, https://doi.org/10.1080/00049182.2017.1336965, 2018.
Grinham, A., Albert, S., Deering, N., Dunbabin, M., Bastviken, D., Sherman, B., Lovelock, C. E., and Evans, C. D.: The importance of small artificial water bodies as sources of methane emissions in Queensland, Australia, Hydrol. Earth Syst. Sci., 22, 5281–5298, https://doi.org/10.5194/hess-22-5281-2018, 2018.
Hirano, T., Segah, H., Kusin, K., Limin, S., Takahashi, H., and Osaki, M.: Effects of disturbances on the carbon balance of tropical peat swamp forests, Glob. Change Biol., 18, 3410–3422, https://doi.org/10.1111/j.1365-2486.2012.02793.x, 2012.
Hooijer, A., Page, S., Jauhiainen, J., Lee, W. A., Lu, X. X., Idris, A., and Anshari, G.: Subsidence and carbon loss in drained tropical peatlands, Biogeosciences, 9, 1053–1071, https://doi.org/10.5194/bg-9-1053-2012, 2012.
Hutchinson, G. L. and Mosier, A. R.: Improved Soil Cover Method for Field Measurement of Nitrous Oxide Fluxes, Soil Sci. Soc. Am. J., 45, 311–316, https://doi.org/10.2136/sssaj1981.03615995004500020017x, 1981.
IPCC: Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands, edited by: Hiraishi, T, Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., and Troxler, T. G., Intergovernmental Panel on Climate Change, Geneva, 2013.
Jeffrey, L. C., Maher, D. T., Chiri, E., Leung, P. M., Nauer, P. A., Arndt, S. K., Tait, D. R., Greening, C., and Johnston, S. G.: Bark-dwelling methanotrophic bacteria decrease methane emissions from trees, Nat. Commun., 12, 2127, https://doi.org/10.1038/s41467-021-22333-7, 2021.
Johnson, A. K. L., Ebert, S. P., and Murray, A. E.: Distribution of coastal freshwater wetlands and riparian forests in the Herbert River catchment and implications for management of catchments adjacent the Great Barrier Reef Marine Park, Environ. Conserv., 26, 229–235, https://doi.org/10.1017/S0376892999000314, 1999.
Kauffman, J. B., Adame, M. F., Arifanti, V. B., Schile-Beers, L. M., Bernardino, A. F., Bhomia, R. K., Donato, D. C., Feller, I. C., Ferreira, T. O., Jesus Garcia, M. del C., MacKenzie, R. A., Megonigal, J. P., Murdiyarso, D., Simpson, L., and Hernández Trejo, H.: Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients, Ecol. Monogr., 90, e01405, https://doi.org/10.1002/ecm.1405, 2020.
Kavehei, E., Iram, N., Rezaei Rashti, M., Jenkins, G. A., Lemckert, C., and Adame, M. F.: Greenhouse gas emissions from stormwater bioretention basins, Ecol. Eng., 159, 106120, https://doi.org/10.1016/j.ecoleng.2020.106120, 2021.
Kettler, T. A., Doran, J. W., and Gilbert, T. L.: Simplified Method for Soil Particle-Size Determination to Accompany Soil-Quality Analyses, Soil Sci. Soc. Am. J., 65, 849–852, https://doi.org/10.2136/sssaj2001.653849x, 2001.
Kiese, R. and Butterbach-Bahl, K.: N2O and CO2 emissions from three different tropical forest sites in the wet tropics of Queensland, Australia, Soil Biol. Biochem., 34, 975–987, 2002.
Kirschke, S., Bousquet, P., Ciais, P., Saunois, M., Canadell, J. G., Dlugokencky, E. J., Bergamaschi, P., Bergmann, D., Blake, D. R., Bruhwiler, L., Cameron-Smith, P., Castaldi, S., Chevallier, F., Feng, L., Fraser, A., Heimann, M., Hodson, E. L., Houweling, S., Josse, B., Fraser, P. J., Krummel, P. B., Lamarque, J. F., Langenfelds, R. L., Le Quéré, C., Naik, V., O'Doherty, S., Palmer, P. I., Pison, I., Plummer, D., Poulter, B., Prinn, R. G., Rigby, M., Ringeval, B., Santini, M., Schmidt, M., Shindell, D. T., Simpson, I. J., Spahni, R., Steele, L. P., Strode, S. A., Sudo, K., Szopa, S., Van Der Werf, G. R., Voulgarakis, A., Van Weele, M., Weiss, R. F., Williams, J. E., and Zeng, G.: Three decades of global methane sources and sinks, Nat. Geosci., 6, 813–823, https://doi.org/10.1038/ngeo1955, 2013.
Knox, S. H., Sturtevant, C., Matthes, J. H., Koteen, L., Verfaillie, J., and Baldocchi, D.: Agricultural peatland restoration: Effects of land-use change on greenhouse gas (CO2 and CH4) fluxes in the Sacramento-San Joaquin Delta, Glob. Change Biol., 21, 750–765, https://doi.org/10.1111/gcb.12745, 2015.
Kögel-Knabner, I., Amelung, W., Cao, Z., Fiedler, S., Frenzel, P., Jahn, R., Kalbitz, K., Kölbl, A., and Schloter, M.: Biogeochemistry of paddy soils, Geoderma, 157, 1–14, https://doi.org/10.1016/J.GEODERMA.2010.03.009, 2010.
Kreuzwieser, J., Buchholz, J., and Rennenberg, H.: Emission of Methane and Nitrous Oxide by Australian Mangrove Ecosystems, Plant Biol., 5, 423–431, https://doi.org/10.1055/s-2003-42712, 2003.
Kristensen, E., Flindt, M. R., Ulomi, S., Borges, A. V., Abril, G., and Bouillon, S.: Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests, Mar. Ecol. Prog. Ser., 370, 53–67, https://doi.org/10.3354/meps07642, 2008.
Krithika, K., Purvaja, R., and Ramesh, R.: Fluxes of methane and nitrous oxide from an Indian mangrove, Curr. Sci., 94, 218–224, 2008.
Kroeger, K. D., Crooks, S., Moseman-Valtierra, S., and Tang, J.: Restoring tides to reduce methane emissions in impounded wetlands: A new and potent Blue Carbon climate change intervention, Sci. Rep., 7, 11914, https://doi.org/10.1038/s41598-017-12138-4, 2017.
Lehner, B. and Döll, P.: Development and validation of a global database of lakes, reservoirs and wetlands, J. Hydrol., 296, 1–22, https://doi.org/10.1016/j.jhydrol.2004.03.028, 2004.
Lenhart, K., Behrendt, T., Greiner, S., Steinkamp, J., Well, R., Giesemann, A., and Keppler, F.: Nitrous oxide effluxes from plants as a potentially important source to the atmosphere, New Phytol., 221, 1398–1408, https://doi.org/10.1111/nph.15455, 2019.
Li, X. and Mitsch, W. J.: Methane emissions from created and restored freshwater and brackish marshes in southwest Florida, USA, Ecol. Eng., 91, 529–536, https://doi.org/10.1016/j.ecoleng.2016.01.001, 2016.
Liu, J. and Lai, D. Y. F.: Subtropical mangrove wetland is a stronger carbon dioxide sink in the dry than wet seasons, Agr. Forest Meteorol., 278, 107644, https://doi.org/10.1016/J.AGRFORMET.2019.107644, 2019.
Maher, D. T., Sippo, J. Z., Tait, D. R., Holloway, C., and Santos, I. R.: Pristine mangrove creek waters are a sink of nitrous oxide OPEN, Nat. Publ. Gr., 6, 25701, https://doi.org/10.1038/srep25701, 2016.
Maietta, C. E., Hondula, K. L., Jones, C. N., and Palmer, M. A.: Hydrological Conditions Influence Soil and Methane-Cycling Microbial Populations in Seasonally Saturated Wetlands, Front. Environ. Sci., 8, 593942, https://doi.org/10.3389/fenvs.2020.593942, 2020.
Mar Lynn, T., Ge, T., Yuan, H., Wei, X., Wu, X., Xiao, K., Kumaresan, D., San Yu, S., Wu, J., and Whiteley, A. S.: Soil Carbon-Fixation Rates and Associated Bacterial Diversity and Abundance in Three Natural Ecosystems, 73, 645–657, https://doi.org/10.1007/s00248-016-0890-x, 2017.
Martiny, J. B. H., Bohannan, B. J. M., Brown, J. H., Colwell, R. K., Fuhrman, J. A., Green, J. L., Horner-Devine, M. C., Kane, M., Krumins, J. A., Kuske, C. R., Morin, P. J., Naeem, S., Øvreås, L., Reysenbach, A. L., Smith, V. H., and Staley, J. T.: Microbial biogeography: Putting microorganisms on the map, Nat. Rev. Microbiol., 4, 102–112, https://doi.org/10.1038/nrmicro1341, 2006.
McGinn, S. M., Turner, D., Tomkins, N., Charmley, E., Bishop-Hurley, G., and Chen, D.: Methane Emissions from Grazing Cattle Using Point-Source Dispersion, J. Environ. Qual., 40, 22–27, https://doi.org/10.2134/jeq2010.0239, 2011.
Melling, L., Goh, K. J., Kloni, A., and Hatano, R.: Is water table the most important factor influencing soil C flux in tropical peatlands, in: Peatlands in Balance, Proceedings of the 14th International Peat Congress, 2012.
Mitsch, W. J., Bernal, B., Nahlik, A. M., Mander, Ü., Zhang, L., Anderson, C. J., Jørgensen, S. E., and Brix, H.: Wetlands, carbon, and climate change, Landsc. Ecol., 28, 583–597, https://doi.org/10.1007/s10980-012-9758-8, 2013.
Morse, J. L. and Marcelo Ardón, E. S. B.: Greenhouse gas fluxes in southeastern U . S . coastal plain wetlands under contrasting land uses, Ecol. Appl., 22, 264–280, 2012.
Musenze, R. S., Werner, U., Grinham, A., Udy, J., and Yuan, Z.: Methane and nitrous oxide emissions from a subtropical estuary (the Brisbane River estuary, Australia), Sci. Total Environ., 472, 719–729, https://doi.org/10.1016/j.scitotenv.2013.11.085, 2014.
National Greenhouse Accounts Factors, Australian Government Department of Industry, Science, Energy and Resources: National Greenhouse Accounts Factors, available at: https://www.industry.gov.au/sites/default/files/2020-10/national-greenhouse-accounts-factors-2020.pdf (last access: 9 August 2021), 2020.
Neubauer, S. C. Global Warming Potential Is Not an Ecosystem Property, Ecosystems, 1–11, https://doi.org/10.1007/s10021-021-00631-x, 2021.
Nieveen, J. P., Campell, D. I., Schipper, L. A., and Blair, I. J.: Carbon exchange of grazed pasture on drained peat soil, Glob. Change Biol., 11, 607–618, https://doi.org/10.1111/j.1365-2486.2005.00929.x, 2005.
Oertel, C., Matschullat, J., Zurba, K., Zimmermann, F., and Erasmi, S.: Greenhouse gas emissions from soils – A review, Chem. Erde, 76, 327–352, https://doi.org/10.1016/j.chemer.2016.04.002, 2016.
Ollivier, Q. R., Maher, D. T., Pitfield, C., and Macreadie, P. I.: Punching above their weight: Large release of greenhouse gases from small agricultural dams, Glob. Change Biol., 25, 721–732, https://doi.org/10.1111/gcb.14477, 2019.
Pangala, S. R., Moore, S., Hornibrook, E. R. C., and Gauci, V.: Trees are major conduits for methane egress from tropical forested wetlands, New Phytol., 197, 524–531, https://doi.org/10.1111/nph.12031, 2013.
Peacock, M., Audet, J., Bastviken, D., Futter, M. N., Gauci, V., Grinham, A., Harrison, J. A., Kent, M. S., Kosten, S., Lovelock, C. E., Veraart, A. J., and Evans, C. D.: Global importance of methane emissions from drainage ditches and canals, Environ. Res. Lett., 16, 044010, https://doi.org/10.1088/1748-9326/abeb36, 2021.
Pinheiro, P. L., Recous, S., Dietrich, G., Weiler, D. A., Schu, A. L., Bazzo, H. L. S., and Giacomini, S. J.: N2O emission increases with mulch mass in a fertilized sugarcane cropping system, Biol. Fert. Soils, 55, 511–523, https://doi.org/10.1007/s00374-019-01366-7, 2019.
Purvaja, R. and Ramesh, R.: Human impacts on methane emission from mangrove ecosystems in India, Reg. Environ. Change, 1, 86–97, 2000.
Purvaja, R., Ramesh, R., and Frenzel, P.: Plant-mediated methane emission from an Indian mangrove, Glob. Change Biol., 10, 1825–1834, https://doi.org/10.1111/j.1365-2486.2004.00834.x, 2004.
Reeves, S., Wang, W., Salter, B., and Halpin, N.: Quantifying nitrous oxide emissions from sugarcane cropping systems: Optimum sampling time and frequency, Atmos. Environ., 136, 123–133, https://doi.org/10.1016/j.atmosenv.2016.04.008, 2016.
Rezaei Rashti, M., Wang, W. J., Harper, S. M., Moody, P. W., Chen, C. R., Ghadiri, H., and Reeves, S. H.: Strategies to mitigate greenhouse gas emissions in intensively managed vegetable cropping systems in subtropical Australia, Soil Res., 53, 475–484, https://doi.org/10.1071/SR14355, 2015.
Rezaei Rashti, M., Wang, W. J., Reeves, S. H., Harper, S. M., Moody, P. W., and Chen, C. R.: Linking chemical and biochemical composition of plant materials to their effects on N2O emissions from a vegetable soil, Soil Biol. Biochem., 103, 502–511, https://doi.org/10.1016/j.soilbio.2016.09.019, 2016.
Rosentreter, J. A., Al-Haj, A. N., Fulweiler, R. W., and Williamson, P.: Methane and Nitrous Oxide Emissions Complicate Coastal Blue Carbon Assessments, Global Biogeochem. Cy., 35, e2020GB006858, https://doi.org/10.1029/2020GB006858, 2021.
Sahrawat, K. L.: Terminal electron acceptors for controlling methane emissions from submerged rice soils, Commun. Soil Sci. Plan., 35, 1401–1413, https://doi.org/10.1081/CSS-120037554, 2004.
Saunois, M., Stavert, A. R., Poulter, B., Bousquet, P., Canadell, J. G., Jackson, R. B., Raymond, P. A., Dlugokencky, E. J., Houweling, S., Patra, P. K., Ciais, P., Arora, V. K., Bastviken, D., Bergamaschi, P., Blake, D. R., Brailsford, G., Bruhwiler, L., Carlson, K. M., Carrol, M., Castaldi, S., Chandra, N., Crevoisier, C., Crill, P. M., Covey, K., Curry, C. L., Etiope, G., Frankenberg, C., Gedney, N., Hegglin, M. I., Höglund-Isaksson, L., Hugelius, G., Ishizawa, M., Ito, A., Janssens-Maenhout, G., Jensen, K. M., Joos, F., Kleinen, T., Krummel, P. B., Langenfelds, R. L., Laruelle, G. G., Liu, L., Machida, T., Maksyutov, S., McDonald, K. C., McNorton, J., Miller, P. A., Melton, J. R., Morino, I., Müller, J., Murguia-Flores, F., Naik, V., Niwa, Y., Noce, S., O'Doherty, S., Parker, R. J., Peng, C., Peng, S., Peters, G. P., Prigent, C., Prinn, R., Ramonet, M., Regnier, P., Riley, W. J., Rosentreter, J. A., Segers, A., Simpson, I. J., Shi, H., Smith, S. J., Steele, L. P., Thornton, B. F., Tian, H., Tohjima, Y., Tubiello, F. N., Tsuruta, A., Viovy, N., Voulgarakis, A., Weber, T. S., van Weele, M., van der Werf, G. R., Weiss, R. F., Worthy, D., Wunch, D., Yin, Y., Yoshida, Y., Zhang, W., Zhang, Z., Zhao, Y., Zheng, B., Zhu, Q., Zhu, Q., and Zhuang, Q.: The Global Methane Budget 2000–2017, Earth Syst. Sci. Data, 12, 1561–1623, https://doi.org/10.5194/essd-12-1561-2020, 2020.
Schindlbacher, A., Zechmeister-Boltenstern, S., and Butterbach-Bahl, K.: Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils, J. Geophys. Res, 109, 17302, https://doi.org/10.1029/2004JD004590, 2004.
Shaaban, M., Peng, Q., Hu, R., Wu, Y., Lin, S., and Zhao, J.: Dolomite application to acidic soils: a promising option for mitigating N2O emissions, Environ. Sci. Pollut. R., 22, 19961–19970, https://doi.org/10.1007/s11356-015-5238-4, 2015.
Solomon, S.: Climate Change: The Physical science Basis, Clim. Chang. Phys. Sci. Basis, available at: http://ci.nii.ac.jp/naid/20001628955/en/ (last access: 13 August 2021), 2007.
Sotomayor, D., Corredor, J. E., and Morell, J. M.: Methane flux from mangrove sediments along the southwestern coast of Puerto Rico, Estuaries, 17, 140–147, 1994.
Timilsina, A., Bizimana, F., Pandey, B., Kailash, R., Yadav, P., Dong, W., and Hu, C.: plants Nitrous Oxide Emissions from Paddies: Understanding the Role of Rice Plants, Plants, 9, 180, https://doi.org/10.3390/plants9020180, 2020.
Ussiri, D. and Lal, R.: Soil emission of nitrous oxide and its mitigation. Springer Science & Business Media, Germany, 378, ISBN 978-94-007-5364-8, 2012.
Veenendaal, E. M., Kolle, O., Leffelaar, P. A., Schrier-Uijl, A. P., Van Huissteden, J., Van Walsem, J., Möller, F., and Berendse, F.: CO2 exchange and carbon balance in two grassland sites on eutrophic drained peat soils, Biogeosciences, 4, 1027–1040, https://doi.org/10.5194/bg-4-1027-2007, 2007.
Whalen, S.: Biogeochemistry of methane exchange between natural wetlands and the atmosphere, Environ. Eng. Sci., 22, 73–94, https://doi.org/10.1089/ees.2005.22.73, 2005.
Whiting, G. J. and Chanton, J. P.: Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration, Tellus B, 53, 521–528, https://doi.org/10.3402/tellusb.v53i5.16628, 2001.
Xu, C., Han, X., Ru, S., Cardenas, L., Rees, R. M., Wu, D., Wu, W., and Meng, F.: Crop straw incorporation interacts with N fertilizer on N2O emissions in an intensively cropped farmland, Geoderma, 341, 129–137, https://doi.org/10.1016/J.GEODERMA.2019.01.014, 2019.
Yvon-Durocher, G., Montoya, J. M., Woodward, G., Jones, J. I., and Trimmer, M.: Warming increases the proportion of primary production emitted as methane from freshwater mesocosms, Glob. Change Biol., 17, 1225–1234, https://doi.org/10.1111/j.1365-2486.2010.02289.x, 2011.
Zaehle, S. and Dalmonech, D.: Carbon-nitrogen interactions on land at global scales: Current understanding in modelling climate biosphere feedbacks, Curr. Opin. Env. Sust., 3, 311–320, https://doi.org/10.1016/j.cosust.2011.08.008, 2011.