the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Tree growth and water-use efficiency at the Himalayan fir treeline and lower altitudes: roles of climate warming and CO2 fertilization
Alpine forests are increasingly exposed to rising temperatures and intensified drought, potentially pushing species beyond their tolerance limits. However, the extent to which rising atmospheric CO2 (Ca) can mitigate these stressors by enhancing tree intrinsic water-use efficiency (iWUE) remains unclear. We investigated the growth and physiological responses of Himalayan fir (Abies spectabilis) using basal area increment (BAI) and δ13C data to track ecophysiological processes over recent decades along an altitudinal gradient in regions with hydrologically distinct regions on the Tibetan Plateau. Significant growth increases were observed at all altitudes in wet regions, while negative growth trends were noted at lower altitudes in dry regions. Climate–growth correlation analysis revealed that growth is primarily constrained by growing season temperatures and spring moisture availability. Tree iWUE increased over time at all altitudes, with a stronger increase in wet regions. Tree growth at lower altitudes in dry stands was negatively related to iWUE, whereas BAI in wet regions was positively associated with iWUE during the post-1965 period. Structural equation modeling indicated that temperature was a key driver of BAI and iWUE at all altitudes in wet regions, while temperature had negative effects on BAI at lower altitudes in dry regions. These results suggest that elevated Ca and temperature can stimulate tree growth in high-altitude forests in wet regions, but the positive effects do not compensate for the negative impacts of reduced water availability at lower altitudes in dry regions. Warming-induced drought stress may thus emerge as a more significant driver of growth compared to increasing Ca levels in comparable alpine forests. Our findings provide critical insights for refining assumptions about CO2 fertilization and climate change effects in ecophysiological models.
- Article
(5116 KB) - Full-text XML
-
Supplement
(2051 KB) - BibTeX
- EndNote
Forests play a crucial role in regulating terrestrial carbon fluxes and influencing the rate of atmospheric CO2 (Ca) increase (Bonan, 2008; Pan et al., 2024), despite facing various climatic and atmospheric changes. Studies have shown that rising Ca has contributed to greater tree growth, driven by the synergistic effects of warming and increased atmospheric CO2 (Saurer et al., 2014; Qi et al., 2015; Silva et al., 2016; Martínez-Sancho et al., 2018; Guo et al., 2022). However, this potential growth benefit is often overshadowed by growth declines induced by warming-related stressors, particularly drought, which has overridden the effects of rising CO2 in the past decade (Peñuelas et al., 2011; Silva and Anand, 2013; Van Der Sleen et al., 2015; Liu et al., 2024; Klesse et al., 2024). Understanding the long-term physiological and growth responses of trees to global changes remains a challenge, especially in climate-sensitive areas (Lindner et al., 2010; Charney et al., 2016; Shestakova et al., 2019; Olano et al., 2023; Sterck et al., 2024).
The Tibetan Plateau, which has experienced a rapid increase in annual mean air temperature at a rate of 0.26 °C per decade over the past 40 years, is warming faster than the global average (Du, 2001). The forests of the Tibetan Plateau are particularly vulnerable to warming due to the amplified temperature increases at higher altitudes (Guo et al., 2018; Sigdel et al., 2018; Mu et al., 2021b; Panthi et al., 2020). Although warmer temperatures have been linked to enhanced vegetation productivity (Piao et al., 2014; Silva et al., 2016; Huang et al., 2017), climate warming increases atmospheric water demand, exacerbating drought stress on plants. Water availability may therefore become increasingly critical for Tibetan Plateau forests under continued warming and rising Ca (Liang et al., 2016b; Silva et al., 2016; Zhao et al., 2023). However, the long-term effects of these changes on tree physiology and growth in Tibetan Plateau forests, particularly in relation to the unprecedented rates of modern warming and increasing Ca, have not been adequately addressed (Xu et al., 2013; Wu et al., 2015; Huang et al., 2017; Panthi et al., 2020).
Tree-ring records provide valuable insights into long-term physiological and growth changes (Bräuning and Mantwill, 2004; McCarroll and Loader, 2004). The isotopic discrimination against 13C that occurs in leaves (i.e., diffusion and carboxylation fractionations) is reflected in the stable carbon isotope ratios (δ13C) of the organic matter produced in a given year. There is a well-established relationship between carbon isotopic discrimination and leaf physiology, such that δ13C is directly related to assimilation rates (A) and stomatal conductance (gs), which together define intrinsic water-use efficiency (iWUE) as the ratio between the two processes (Farquhar et al., 1982, 1989). Global studies using tree-ring δ13C data have shown widespread increases in iWUE due to enhanced photosynthesis and carbon availability in response to rising Ca (Saurer et al., 2004; Wang et al., 2012; Keenan et al., 2013; Peñuelas et al., 2013; Frank et al., 2015). However, climatic factors, such as temperature and precipitation, may also influence iWUE and reduce the potential CO2 fertilization effects on radial growth (Frank et al., 2015; Guerrieri et al., 2019; Liu et al., 2019; Wang et al., 2020; Zhang et al., 2018). Furthermore, the spatial variability in climate warming and decreasing moisture availability may be more influential in driving tree growth than changes in Ca in cold mountain forests (Salzer et al., 2009; Pu et al., 2021). Recent studies have highlighted contrasting physiological strategies among plant species and altitudes in response to environmental changes (Klein, 2014; Garcia-Forner et al., 2016; Martínez-Vilalta and Garcia-Forner, 2017; Fang et al., 2020; Wang et al., 2020). Therefore, differences in water-use efficiency and ecophysiological strategies across species and regions underscore the importance of shifting from global studies to more localized, species-specific approaches for assessing the long-term effects of warming temperatures and rising Ca (Frank et al., 2015; Martínez-Sancho et al., 2018; Voltas et al., 2020).
In this study, we present annually resolved iWUE and basal area increment (BAI) data for Himalayan fir (Abies spectabilis), a widely distributed conifer species of the Tibetan Plateau. Although some studies have explored the relationship between iWUE and growth of Himalayan fir on the Tibetan Plateau (Huang et al., 2017; Panthi et al., 2020; Wang et al., 2020), they have typically focused on single locations. Here, we examine a broader set of stands distributed along altitudinal gradients and in both wet and dry regions of the Tibetan Plateau, aiming to identify the climatic conditions under which trees exhibit vulnerability. Our specific objectives are (i) to explore whether rising Ca and climate changes have induced region-specific and altitude-specific changes in tree growth and physiological parameters, (ii) to determine the extent to which changes in climate and iWUE are related to radial growth in the study area, and (iii) to assess the physiological adjustments of trees to rising temperature and increased atmospheric CO2 over the study period.
2.1 Site conditions and tree species
The study was conducted in the southern and southeastern regions of the Xizang Province, located on the southern and southeastern Tibetan Plateau, characterized by a typical monsoon climate. The study sites spanned a broad range across the southern and southeastern Tibetan Plateau (Fig. 1). Meteorological data, including mean temperature and precipitation, were obtained from two meteorological stations of the China Meteorological Administration (Fig. 1) for the study period (1950–2010s). Instrumental records showed a mean annual temperature of 0.23 °C and an average total precipitation of 429 mm in Pali (wet region) in recent decades, while the mean temperature is 2.7 °C and the average total precipitation is only 282 mm in Dingri (dry region) in recent decades (Fig. S1 in the Supplement). Additionally, we retrieved climate data, including monthly mean temperature, monthly precipitation, and 3-month Standardized Precipitation Evapotranspiration Index (SPEI) data, from the Climate Research Unit (CRU; University of East Anglia) TS Version 4.01 from the climate explorer (http://climexp.knmi.nl, last access: 28 October 2024). The SPEI data were calculated based on the climate data from the CRU TS Version 4.01 with a spatial resolution of 0.5°×0.5° over the period from 1900–2014. The regional mean values of SPEI were calculated over the grids within the region 27.75°–28.25° N, 88.75°–89.25° E for the wetter site (SYD) and the region 27.95°–28.45° N, 86.75°–87.25° E for the drier site (DJ). The regional mean climate data reveal a significant warming trend in the study area over the past century, with the rate of temperature increase accelerating from 0.007 °C yr−1 (1901–1964) to 0.032 °C yr−1 (1965–2013) at the drier site (DJ) and from 0.008–0.023 °C yr−1 at the wetter site (SYD) (Fig. S2a in the Supplement). In contrast, annual precipitation remained stable from 1901 (Fig. S2b), leading to an overall decline in SPEI at both sites (Fig. S2c).
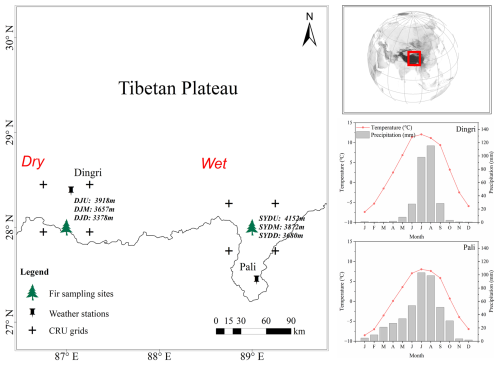
Figure 1Location of the study sites and weather stations for Himalayan fir on the Tibetan Plateau. Climate diagrams are based on meteorological records from the Dingri (28°38′ N, 87°5′ E; 4300 m) and Pali (27°44′ N, 89°5′ E; 4300 m) weather stations for the period 1965–2013.
Himalayan fir (Abies spectabilis) is a cold-tolerant species native to the high-altitude montane forests of the Himalayas. It has a wide altitudinal distribution ranging from 2800 m a.s.l. (meters above sea level) to the upper treeline. The species is found across the central and western Himalayas, with a significant presence on the southwestern and southern Tibetan Plateau (Panthi et al., 2020).
2.2 Field sampling and tree-ring-width chronologies
The tree-ring samples used in this study were collected from healthy Himalayan fir trees growing at altitudes ranging from 3378–4557 m a.s.l. in the southern and southeastern Tibetan Plateau (Table S1 in the Supplement and Fig. 1). These sites were undisturbed by human activities during the study period. Tree-ring increment cores were extracted from trees along an altitudinal gradient in both dry (Dingjie) and wet (Shangyadong) regions. The cores were air-dried indoors, mounted on wooden slats, and polished with progressively finer sandpaper up to 1000 grit until the tree-ring boundaries became clearly visible. Using a microscope, tree rings from each sample were cross-dated by comparing ring patterns among samples. We measured tree-ring widths using a LINTAB 6 measuring system, with a resolution of 0.001 mm. Visual cross-dating was verified with COFECHA software (Holmes and Kozinn, 1983). All cross-dated ring widths were processed using ARSTAN software to standardize the ring-width series, employing a negative exponential or linear growth curve to remove non-climatic signals. The detrended index series were then merged using the biweight robust mean method to create a standard (STD) chronology for each forest stand. For each tree-ring value, tree-ring width was converted into basal area increment (BAI; cm2 yr−1) using the following formula: , where R is the radius of the tree and n is the year of tree-ring formation. The raw basal area increment (BAI) series were used to assess long-term growth trends, as BAI is less susceptible to biological trends and provides a more direct measure of stem biomass compared to tree-ring-width data (Franco and Fares, 2008; Martinez-Sancho et al., 2018; Yang et al., 2022). To further minimize age-related effects, we excluded the early growth period from both trend analysis and isotope measurements. For climate–growth relationship analysis, we applied standard detrending procedures to BAI series using either a negative exponential or a linear function. The calculation and detrending of BAI were performed using the R package dplR (Bunn, 2008).
2.3 Stable carbon isotope (δ13C) and intrinsic water-use efficiency (iWUE)
Five cores from different trees were selected at each forest stand, ensuring clear and continuous ring boundaries with no missing rings. The annual rings from the five samples were pooled by year to produce a single composite isotope series for each forest stand (Table S2 in the Supplement). The wood material was ground using a centrifugal mill to ensure homogeneity and efficiency in α-cellulose extraction. The α-cellulose was extracted from the annual tree rings following standard methods (Loader et al., 1997). To maximize homogeneity, the cellulose was treated in an ultrasound unit in a hot water bath (JY92-2D, Scientz Industry, Ningbo, China) to break down the cellulose (Laumer et al., 2009). The α-cellulose was then freeze-dried for 72 h using a vacuum freeze-dryer (Labconco Corporation, Kansas City, MO, USA) prior to isotope analysis. The δ13C values were determined using an element analyzer (Flash EA 1112; Bremen, Germany) coupled with an isotope-ratio mass spectrometer (Delta-plus, Thermo Electron Corporation, Bremen, Germany) at the State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences. The analytical errors (standard deviations) for the isotope measurements were less than 0.05 ‰ for δ13C. Calibration was performed using the International Atomic Energy Agency (IAEA) standards, USGS-24 (graphite) and IAEA-CH3 (cellulose). All δ13C values are expressed relative to their respective standards (Vienna Pee Dee Belemnite for carbon isotopes and Vienna Standard Mean Ocean Water for oxygen isotopes).
The formula used to calculate δ13C is
where R represents the ratio of 13C to 12C and Rsample and Rstandard are the R values of the samples and the standard, respectively.
To accurately obtain tree-ring δ13C, the climate change effect, i.e., the increasing trend in atmospheric CO2 concentration, was removed. The atmospheric CO2 concentration data were derived from a combination of the reconstructed values (period 1900–2003) using ice cores (Mccarroll and Loader, 2004; Boucher et al., 2014) and the direct observations of CO2 concentration for the period 2004–2013, which were obtained from the Mauna Loa Observatory of America (http://www.esrl.noaa.gov/gmd/obop/mlo/, last access: 1 December 2023). The carbon isotope fractionation sequence in the tree rings was then calculated using the following equations (Farquhar et al., 1989):
where 13Cp and 13Ca were 13C values of plant photosynthetic products and atmospheric CO2, respectively. The ratio of Ci to Ca was calculated using
where Ci and Ca represent the concentrations of CO2 in the leaves and atmosphere, respectively. a and b are constants representing CO2 isotope fractionation during stomatal diffusion (4.4 ‰) and RuBP enzyme carboxylation (27 ‰). iWUE was then estimated using Ci and Ca following Ehleringer and Cerling (1995):
where 1.6 is the ratio of diffusivities of water and CO2 in air.
2.4 Statistical analyses
Linear trends for annual climatic variables and tree growth variables were calculated using least-squares regressions. The relationship between the Ci and Ca trends obtained from δ13C and the three theoretical gas-exchange scenarios was quantified using root-mean-square error (RMSE) and mean absolute error (MAE) for each study site. Mann–Kendall trend tests were conducted to identify the most recent significant warming period (1965 to the present).
To quantify the relationships between climatic factors, intrinsic water-use efficiency (iWUE), and basal area increment (BAI) over the past 4 decades, we implemented a piecewise structural equation modeling (pSEM) approach. The analysis was performed in R version 4.1 (R Core Team, 2022) using the pSEM package (Lefcheck, 2016), which enables the integration of mixed-effects models within an SEM framework. We used a linear mixed-effects modeling approach (Nakagawa and Schielzeth, 2013) to partition variance contributions (R2) from both fixed effects and random effects on BAI and iWUE. This approach allowed us to assess direct and indirect pathways while accounting for potential hierarchical dependencies in the data. Model adequacy was evaluated using Fisher's C statistic, with a non-significant result (p>0.05) indicating acceptable model fit (Shipley, 2009). This analytical framework provided a robust assessment of how climatic drivers influence tree growth both directly and indirectly through physiological adjustments in iWUE.
3.1 Temporal variability in BAI, iWUE, and cellulose stable carbon isotopes
The basal area increment (BAI) of Himalayan fir exhibited a significant increasing trend at the treeline in the dry region and at all altitudes in the wet region after 1965 (Fig. 2). In contrast, no significant trend was detected at the middle altitude, and a decreasing growth trend was observed at the lower altitudes in the dry region (Fig. 2b). Similarly, the intrinsic water-use efficiency (iWUE) of Himalayan fir increased significantly, with a steep rise after 1965 (Fig. 3b). The carbon isotopic composition (δ13C) of tree rings showed a clear decreasing trend over recent decades, reflecting the rising atmospheric CO2 concentration (Ca) across all altitudes in both dry and wet regions (Fig. S3 in the Supplement). Our results indicate that tree growth increased with the rising iWUE in wet regions, while the relationship between iWUE and growth shifted from a significant positive correlation to a negative correlation as altitude decreased in the dry regions (Fig. 4).
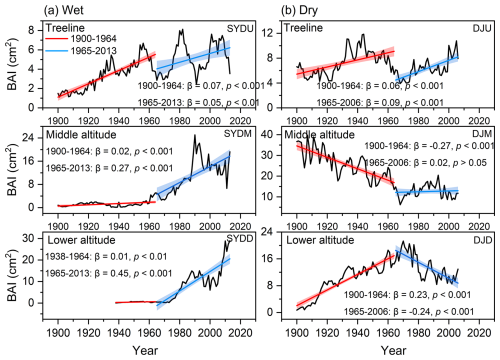
Figure 2Temporal trends in basal area increment (BAI) for Himalayan fir at different altitudes during the period 1900–2010s. The solid lines depict piecewise linear regression models, with a breakpoint at 1965. The model statistics include the slope of BAI (β, cm2 yr−1), coefficient of determination (R2), and associated p values. The shaded regions denote 95 % confidence intervals for the regression fits.
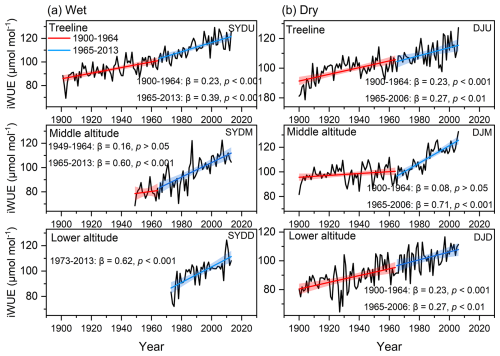
Figure 3Temporal trends in intrinsic water-use efficiency (iWUE) during the period 1900–2010s for Himalayan fir at different altitudes. The solid lines depict piecewise linear regression models, with a breakpoint at 1965. The model statistics include the slope of BAI (β, cm2 yr−1), coefficient of determination (R2), and associated p values. The shaded regions denote 95 % confidence intervals for the regression fits.
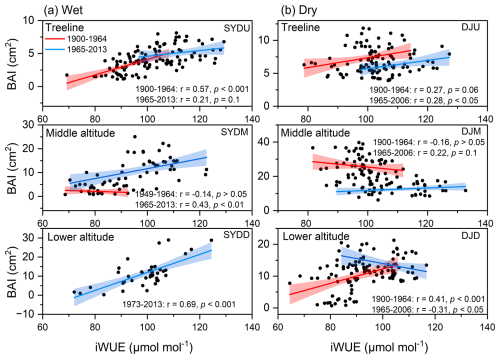
Figure 4Long-term relationships between intrinsic water-use efficiency (iWUE) and basal area increment (BAI) of Himalayan fir at different altitudes across two hydrologically distinct sites. Linear regression results for each period are shown, along with the explained variance (R2) and significance levels (p values). Shaded areas represent 95 % confidence intervals.
3.2 Climate responses of BAI
The BAI of Himalayan fir exhibited region-specific climate sensitivity, with consistent responses observed in the wet region and varying responses along the altitude gradient in the dry region. In the wet region, tree growth showed a significant positive correlation with both the previous autumn temperature and the growing season (June–September) temperature at all altitudes (Fig. 5a). In the dry region, however, tree growth was positively correlated with the previous autumn and growing season temperature at the treeline, while a significant negative correlation was observed at lower altitudes (Fig. 5a). Additionally, tree growth in the wet region was positively correlated with spring precipitation, whereas, in the dry region, spring precipitation was negatively correlated with tree growth at lower altitudes (Fig. 5b). The moisture-related variables (relative humidity and SPEI) exhibited generally negative correlations with tree growth in the wet region but positive correlations in the dry region, particularly at mid- and low-altitude stands (Fig. 5c and d). Notably, when BAI chronologies were constructed using all dated core samples, rather than only those selected for isotope analysis, the resulting climate response patterns remained consistent with the above findings (Fig. S4 in the Supplement).
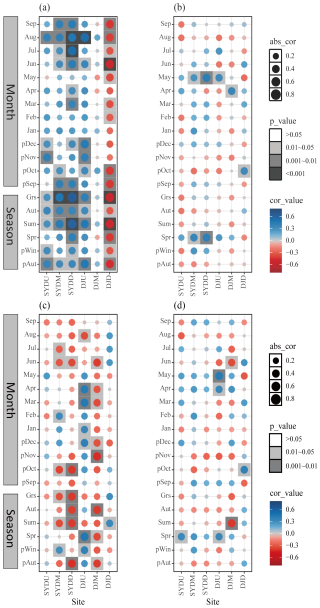
Figure 5Pearson correlation coefficients between basal area increment (BAI) and climatic variables, including (a) mean temperature, (b) total precipitation, (c) relative humidity, and (d) Standardized Precipitation Evapotranspiration Index (SPEI), on both monthly and seasonal scales for Himalayan fir during the period 1965–2013. It should be noted that the BAI chronologies were derived exclusively from samples used for isotope measurements. “Grs” denotes the growing season (June–September). “Cor_value” and “abs_value” represent the correlation coefficient value and the absolute value of the correlation, respectively.
3.3 Factors regulating changes in BAI
Multiple regression models identified significant contributions of temperature, precipitation/moisture availability, and iWUE to interannual variations in tree growth over the past decades (Fig. 6). In the wet region, regression models identified previous autumn temperature (Tgrs) and growing season temperature (Tpaut) as the dominant explanatory variables for radial growth variability, with supplementary contributions from iWUE and spring precipitation at lower-altitude sites (Fig. 6). In the dry region, tree growth was primarily influenced by spring moisture availability and temperature (Fig. 6).
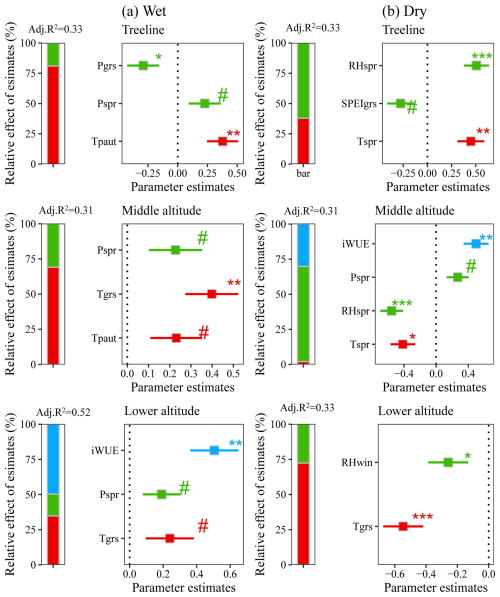
Figure 6Effects of multiple environmental factors on fir growth at different altitudes. The symbols #, *, **, and *** denote significance levels at p<0.1, p<0.05, p<0.01, and p<0.001, respectively. Abbreviations: Tspr=spring mean temperature; Tgrs=growing season mean temperature (June–September); RHspr=spring relative humidity; RHgrs=growing season relative humidity (June–September); RHwin=winter relative humidity; SPEIspr=spring Standardized Precipitation Evapotranspiration Index; SPEIgrs=growing season Standardized Precipitation Evapotranspiration Index (June–September).
Overall, the climatic and physiological factors accounted for 25 %–58 % of the observed growth variation, as quantified through piecewise structural equation modeling (pSEM) (Fig. 7 and Table S3 in the Supplement). The pSEM framework elucidated distinct mechanistic pathways governing basal area increment (BAI) across climatic zones. In the wet region, Tgrs emerged as the principal determinant of BAI (Fig. 7a), while iWUE exerted significant positive direct effects on BAI at lower altitudes (Fig. 7). Notably, Tgrs demonstrated a significant positive relationship with iWUE in these humid forests (Fig. 7). Dry-region stands exhibited contrasting responses, with Tgrs and spring temperature (Tspr) showing significant negative associations with BAI at mid- and low-altitude sites. Conversely, positive growth responses to spring moisture availability (RHspr and Pspr) were observed at the treeline and middle altitudes (Fig. 7), highlighting water limitation as the primary growth constraint in these environments.
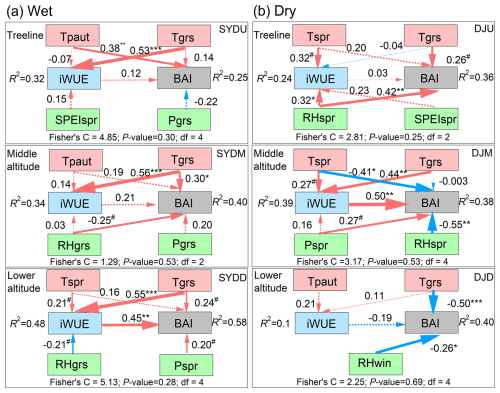
Figure 7Piecewise structural equation meta-model used to assess the influences of climatic factors on basal area increment (BAI) and intrinsic water-use efficiency (iWUE) of Himalayan fir during the period 1965–2010s at different altitudes. Numbers adjacent to each arrow indicate the standardized regression coefficients for each path. Arrow thickness represents the strength of the effect, while color indicates the direction (red = positive, blue = negative). Solid arrows denote significant effects, whereas dashed arrows denote non-significant effects. Symbols #, *, **, and *** indicate significance levels at p<0.1, p<0.05, p<0.01, and p<0.001, respectively. Abbreviations: Tpaut = previous autumn mean temperature; Tsum = summer mean temperature; Tspr = spring mean temperature; Tgrs = growing season mean temperature (June–September); RHspr = spring relative humidity; RHgrs = growing season relative humidity (June–September); RHwin = winter relative humidity; SPEIspr = spring Standardized Precipitation Evapotranspiration Index; SPEIgrs = growing season Standardized Precipitation Evapotranspiration Index (June–September).
4.1 Tree growth and its climatic responses
Our study reveals a distinct pattern of tree growth across different regions and altitudes on the Tibetan Plateau, with a strong acceleration of growth in the wet region and at higher altitudes in the dry region, contrasting with decreasing growth trends at lower altitudes in the dry region. These results diverge from the widely observed global trend of declining tree growth and increased forest mortality due to high temperatures and drought, particularly in other parts of the world (Allen et al., 2010; Hartmann et al., 2018; Mirabel et al., 2023). While numerous studies have documented the detrimental effects of warming and drought on forest ecosystems, particularly in the northeastern Tibetan Plateau (Liang et al., 2016a; Wang et al., 2018), our findings suggest that the Tibetan Plateau, particularly in its southeastern and southern regions, is undergoing a phase of warming and humidification that has benefited tree growth (Shi et al., 2020; Mu et al., 2021a; Guo et al., 2022).
The acceleration of growth in the wet regions aligns with the well-established finding that increased temperature can enhance tree productivity in humid climates (Liang et al., 2016b; Silva et al., 2016; Wang et al., 2023), which is further confirmed by our results showing a positive correlation between growth and growing season temperature. However, this contrasts with the negative or stable growth trends observed in the dry region, particularly at lower altitudes, where warming-induced drought stress appears to inhibit growth. Our study extends existing research by highlighting that tree growth in moisture-limited ecosystems (such as the dry regions) is more constrained by water availability than by warming. This reinforces the growing body of literature suggesting that CO2 fertilization effects are more likely to occur in temperature-limited ecosystems (Körner, 2015), whereas moisture-limited regions are less responsive to increased Ca (Wu et al., 2015).
This research provides novel insights into how specific regional climatic shifts may lead to divergent growth responses in montane forests. In contrast to the increasing mortality rates and growth decline observed in other high-altitude forest systems globally (Linares and Camarero, 2012; Lévesque et al., 2014), our study suggests that areas undergoing warming and increased moisture availability could experience enhanced tree growth, provided drought stress remains manageable.
4.2 Spatial heterogeneity of tree growth–iWUE relationships
Our findings reveal a consistent decline in δ13C values in Himalayan fir, corresponding to a marked increase in intrinsic water-use efficiency (iWUE), particularly in mesic regions and at treeline sites in arid zones. This trend aligns with global observations of enhanced iWUE under rising atmospheric CO2 (Ca) (Frank et al., 2015; Huang et al., 2017; Guerrieri et al., 2019). However, our results demonstrate greater complexity in the iWUE response of Himalayan fir than previously documented, with pronounced site- and altitude-specific variability. Specifically, at the mid- and low-altitude sites in the arid region, limited moisture availability appears to constrain iWUE gains despite rising Ca, whereas the forest stands with more moisture availability, including wet sites and the treeline site of the drier region, exhibit more pronounced iWUE increases. These findings provide a physiological framework for understanding how trees adjust water-use strategies under concurrent increases in CO2 and temperature.
While elevated Ca is frequently associated with enhanced photosynthesis and iWUE (Cole et al., 2010; Brienen et al., 2011; Saurer et al., 2014), the observed increase in intrinsic water-use efficiency (iWUE) does not necessarily correspond to enhanced radial growth, as evidenced by the non-significant relationship between basal area increment (BAI) and iWUE (Andreu-Hayles et al., 2011; Girardin et al., 2016; Reed et al., 2018), particularly in the dry regions (Peñuelas et al., 2011; Franks et al., 2013; Silva and Anand, 2013; Peters et al., 2018). This pattern suggests a drought-tolerance strategy that optimizes the trade-off between transpiration and carbon assimilation, highlighting the importance of local-scale ecophysiological adaptations to climate change (Aranda et al., 2000; Hereş etal., 2014; Panthi et al., 2020). The negative correlation between BAI and iWUE at the low-altitude stands in the dry region supports the idea that drought stress can offset the benefits of CO2 fertilization (Granda et al., 2014; Fang et al., 2020). Moreover, previous investigations have revealed altitudinal divergence in the physiological mechanisms driving iWUE enhancement: while treeline populations primarily achieved increased iWUE through photosynthetic enhancement, populations at lower elevations predominantly relied on stomatal conductance reduction (Pu and Lyu, 2023). In this study, divergent intercellular CO2 concentrations (Ca) across sites suggest plasticity in leaf gas exchange (Fig. S5 in the Supplement), demonstrating that trees actively regulate physiological traits in response to rising Ca, rather than responding passively (Ainsworth and Rogers, 2007; Walker et al., 2015; Voelker et al., 2016). These adjustments reflect an adaptive capacity to balance carbon gain with water conservation under changing climatic conditions.
These findings emphasize the need for meso- to local-scale investigations to unravel the interactive effects of Ca, temperature, and moisture on tree physiology. This is particularly critical in high-altitude ecosystems such as the Himalayas, where climatic gradients create complex, non-linear responses in tree growth and iWUE (Körner, 2003; Salzer et al., 2009). Future research should prioritize mechanistic models that integrate these spatial and temporal variations to improve predictions of forest dynamics under climate change.
In summary, our study provides novel insights into the ecophysiological and growth responses of Himalayan fir to climate change on the Tibetan Plateau. By examining the effects of rising atmospheric CO2, temperature, and drought across different regions and altitudes, we have shown that climate change has diverse effects on tree growth, with moisture availability being a critical limiting factor at lower altitudes. Our findings underscore the importance of considering spatial and regional differences when assessing the impacts of climate change on forest ecosystems and highlight the complex interactions between temperature, moisture, and CO2 in shaping tree growth patterns. These insights are crucial for refining our understanding of forest dynamics and carbon cycling in montane ecosystems and for predicting the future trajectory of high-altitude forests under climate change.
Data will be made available on request.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-4993-2025-supplement.
LL acquired the funding and designed the study. XP processed the tree-ring samples, conducted the analysis, and wrote the first draft. LL and XP interpreted the results, revised the article, and contributed to writing. Both authors reviewed and approved the final submission.
The contact author has declared that neither of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Treeline ecotones under global change: linking spatial patterns to ecological processes”. It is not associated with a conference.
This research was funded by the Natural Science Foundation of China (grant no. 42271074). We are grateful to Qi-Bin Zhang for his supervision on the research. We also want to thank Hongyan Qiu for her kind assistance with the cross-dating of the tree-ring samples.
This research has been supported by the National Natural Science Foundation of China (grant no. 42271074).
This paper was edited by Matteo Garbarino and reviewed by two anonymous referees.
Ainsworth, E. A. and Rogers, A.: The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions, Plant Cell Environ., 30, 258–270, https://doi.org/10.1111/j.1365-3040.2007.01641.x, 2007.
Allen, C. D., Macalady, A. K., Chenchouni, H., Bachelet, D., McDowell, N., Vennetier, M., Kitzberger, T., Rigling, A., Breshears, D. D., Hogg, E. H., Gonzalez, P., Fensham, R., Zhang, Z., Castro, J., Demidova, N., Lim, J. H., Allard, G., Running, S. W., Semerci, A., and Cobb, N.: A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests, Forest. Ecol. Manag., 259, 660–684, https://doi.org/10.1016/j.foreco.2009.09.001, 2010.
Andreu-Hayles, L., Planells, O., Gutierrez, E., Muntan, E., Helle, G., Anchukaitis, K. J., and Schleser, G. H.: Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests, Glob. Change Biol., 17, 2095–2112, https://doi.org/10.1111/j.1365-2486.2010.02373.x, 2011.
Aranda, I., Gil, L., and Pardos, J. A.: Water relations and gas exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. in a mixed stand at their southern limit of distribution in Europe, Trees-Struct. Funct., 14, 344–352, https://doi.org/10.1007/s004680050229, 2000.
Bonan, G. B.: Forests and climate change: Forcings, feedbacks, and the climate benefits of forests, Science, 320, 1444–1449, https://doi.org/10.1126/science.1155121, 2008.
Boucher, E., Guiot, J., Daux, V., Danis, P. A., and Dussouillez, P.: An inverse modeling approach for tree-ring-based climate reconstructions under changing atmospheric CO2 concentrations, Biogeosciences, 10, 3245–3258, https://doi.org/10.5194/bg-11-3245-2014, 2014.
Bräuning, A. and Mantwill, B.: Summer temperature and summer monsoon history on the Tibetan plateau during the last 400 years recorded by tree rings, Geophys. Res. Lett., 31, L24205, https://doi.org/10.1029/2004gl020793, 2004.
Brienen, R. J. W., Wanek, W., and Hietz, P.: Stable carbon isotopes in tree rings indicate improved water use efficiency and drought responses of a tropical dry forest tree species, Trees-Struct. Funct., 25, 103–113, https://doi.org/10.1007/s00468-010-0474-1, 2011.
Bunn, A. G.: A dendrochronology program library in R (dplR), Dendrochronologia, 26, 115–124, https://doi.org/10.1016/j.dendro.2008.01.002, 2008.
Charney, N. D., Babst, F., Poulter, B., Record, S., Trouet, V. M., Frank, D., Enquist, B. J., and Evans, M. E.: Observed forest sensitivity to climate implies large changes in 21st century North American forest growth, Ecol. Lett., 19, 1119–1128, https://doi.org/10.1111/ele.12650, 2016.
Cole, C. T., Anderson, J. E., Lindroth, R. L., and Waller, D. M.: Rising concentrations of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremuloides), Glob. Change Biol., 16, 2186–2197, https://doi.org/10.1111/j.1365-2486.2009.02103.x, 2010.
Du, J.: Change of temperature in Tibetan Plateau from 1961 to 2000, Acta Geographica Sinica, 56, 682–690, https://doi.org/10.11821/xb200106007, 2001.
Ehleringer, J. R. and Cerling, T. E.: Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentration in plants, Tree Physiol., 15, 105–111, https://doi.org/10.1093/treephys/15.2.105, 1995.
Fang, O., Qiu, H., and Zhang, Q.-B.: Species-specific drought resilience in juniper and fir forests in the central Himalayas, Ecol. Indic., 117, 106615, https://doi.org/10.1016/j.ecolind.2020.106615, 2020.
Farquhar, G. D., Ehleringer, J. R., and Hubick, K. T.: Carbon Isotope Discrimination and Photosynthesis, Annu. Rev. Plant Phys., 40, 503–537, https://doi.org/10.1146/annurev.pp.40.060189.002443, 1989.
Farquhar, G. D., Oleary, M. H., and Berry, J. A.: On the Relationship between Carbon Isotope Discrimination and the Inter-Cellular Carbon-Dioxide Concentration in Leaves, Aust. J. Plant. Physiol., 9, 121–137, https://doi.org/10.1071/PP9820121, 1982.
Franco, B. and Fares, Q.: A theory-driven approach to tree-ring standardization: defining the biological trend from expected basal area increment, Tree Ring Res., 64, 81-96, https://doi.org/10.3959/2008-6.1, 2008.
Frank, D. C., Poulter, B., Saurer, M., Esper, J., Huntingford, C., Helle, G., Treydte, K., Zimmermann, N. E., Schleser, G. H., Ahlstrom, A., Ciais, P., Friedlingstein, P., Levis, S., Lomas, M., Sitch, S., Viovy, N., Andreu-Hayles, L., Bednarz, Z., Berninger, F., Boettger, T., D'Alessandro, C. M., Daux, V., Filot, M., Grabner, M., Gutierrez, E., Haupt, M., Hilasvuori, E., Jungner, H., Kalela-Brundin, M., Krapiec, M., Leuenberger, M., Loader, N. J., Marah, H., Masson-Delmotte, V., Pazdur, A., Pawelczyk, S., Pierre, M., Planells, O., Pukiene, R., Reynolds-Henne, C. E., Rinne, K. T., Saracino, A., Sonninen, E., Stievenard, M., Switsur, V. R., Szczepanek, M., Szychowska-Krapiec, E., Todaro, L., Waterhouse, J. S., and Weigl, M.: Water-use efficiency and transpiration across European forests during the Anthropocene, Nat. Clim. Chang., 5, 579–583, https://doi.org/10.1038/nclimate2614, 2015.
Franks, P. J., Adams, M. A., Amthor, J. S., Barbour, M. M., Berry, J. A., Ellsworth, D. S., Farquhar, G. D., Ghannoum, O., Lloyd, J., McDowell, N., Norby, R. J., Tissue, D. T., and von Caemmerer, S.: Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century, New Phytol., 197, 1077–1094, https://doi.org/10.1111/nph.12104, 2013.
Garcia-Forner, N., Adams, H. D., Sevanto, S., Collins, A. D., Dickman, L. T., Hudson, P. J., Zeppel, M. J., Jenkins, M. W., Powers, H., Martinez-Vilalta, J., and McDowell, N. G.: Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation, Plant Cell Environ., 39, 38–49, https://doi.org/10.1111/pce.12588, 2016.
Girardin, M. P., Bouriaud, O., Hogg, E. H., Kurz, W., Zimmermann, N. E., Metsaranta, J. M., de Jong, R., Frank, D. C., Esper, J., Buntgen, U., Guo, X. J., and Bhatti, J.: No growth stimulation of Canada's boreal forest under half-century of combined warming and CO2 fertilization522, P. Natl. Acad. Sci. USA, 113, E8406–E8414, https://doi.org/10.1073/pnas.1610156113, 2016.
Granda, E., Rossatto, D. R., Camarero, J. J., Voltas, J., and Valladares, F.: Growth and carbon isotopes of Mediterranean trees reveal contrasting responses to increased carbon dioxide and drought, Oecologia, 174, 307–317, https://doi.org/10.1007/s00442-013-2742-4, 2014.
Guerrieri, R., Belmecheri, S., Ollinger, S. V., Asbjornsen, H., Jennings, K., Xiao, J., Stocker, B. D., Martin, M., Hollinger, D. Y., Bracho-Garrillo, R., Clark, K., Dore, S., Kolb, T., Munger, J. W., Novick, K., and Richardson, A. D.: Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency, P. Natl. Acad. Sci. USA, 116, 16909–16914, https://doi.org/10.1073/pnas.1905912116, 2019.
Guo, M. M., Zhang, Y. D., Wang, X. C., Gu, F. X., and Liu, S. R.: The responses of dominant tree species to climate warming at the treeline on the eastern edge of the Tibetan Plateau, Forest Ecol. Manag., 425, 21–26, https://doi.org/10.1016/j.foreco.2018.05.021, 2018.
Guo, Y., Zhang, L., Yang, L., Shen, W., Pan, Y., Wright, I. J., Luo, Y., and Luo, T.: Enhanced leaf turnover and nitrogen recycling sustain CO2 fertilization effect on tree-ring growth, Nat. Ecol. Evol., 6, 1271–1278, https://doi.org/10.1038/s41559-022-01811-1, 2022.
Hartmann, H., Moura, C. F., Anderegg, W. R. L., Ruehr, N. K., Salmon, Y., Allen, C. D., Arndt, S. K., Breshears, D. D., Davi, H., Galbraith, D., Ruthrof, K. X., Wunder, J., Adams, H. D., Bloemen, J., Cailleret, M., Cobb, R., Gessler, A., Grams, T. E. E., Jansen, S., Kautz, M., Lloret, F., and O'Brien, M.: Research frontiers for improving our understanding of drought-induced tree and forest mortality, New Phytol., 218, 15–28, https://doi.org/10.1111/nph.15048, 2018.
Hereş, A. M., Voltas, J., Lopez, B. C., and Martinez-Vilalta, J.: Drought-induced mortality selectively affects Scots pine trees that show limited intrinsic water-use efficiency responsiveness to raising atmospheric CO2, Funct. Plant Biol., 41, 244–256, https://doi.org/10.1071/Fp13067, 2014.
Holmes, R. L. and Kozinn, W. P.: Pneumonia and Bacteremia Associated with Hemophilus-Influenzae Serotype-D, J. Clin. Microbiol., 18, 730–732, https://doi.org/10.1128/Jcm.18.3.730-732.1983, 1983.
Huang, R., Zhu, H. F., Liu, X. H., Liang, E. Y., Griessinger, J., Wu, G. J., Li, X. X., and Brauning, A.: Does increasing intrinsic water use efficiency (iWUE) stimulate tree growth at natural alpine timberline on the southeastern Tibetan Plateau?, Global Planet Change, 148, 217–226, https://doi.org/10.1016/j.gloplacha.2016.11.017, 2017.
Keenan, T. F., Hollinger, D. Y., Bohrer, G., Dragoni, D., Munger, J. W., Schmid, H. P., and Richardson, A. D.: Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise, Nature, 499, 324–327, https://doi.org/10.1038/nature12291, 2013.
Klein, T.: The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours, Funct. Ecol., 28, 1313–1320, https://doi.org/10.1111/1365-2435.12289, 2014.
Klesse, S., Peters, R. L., Alfaro-Sánchez, R., Badeau, V., Baittinger, C., Battipaglia, G., Bert, D., Biondi, F., Bosela, M., Budeanu, M., Čada, V., Camarero, J. J., Cavin, L., Claessens, H., Cretan, A.-M., Čufar, K., de Luis, M., Dorado-Liñán, I., Dulamsuren, C., Espelta, J. M., Garamszegi, B., Grabner, M., Gricar, J., Hacket-Pain, A., Hansen, J. K., Hartl, C., Hevia, A., Hobi, M., Janda, P., Jump, A. S., Kašpar, J., Kazimirović, M., Keren, S., Kreyling, J., Land, A., Latte, N., Lebourgeois, F., Leuschner, C., Lévesque, M., Longares, L. A., del Castillo, E. M., Menzel, A., Merela, M., Mikoláš, M., Motta, R., Muffler, L., Neycken, A., Nola, P., Panayotov, M., Petritan, A. M., Petritan, I. C., Popa, I., Prislan, P., Levanič, T., Roibu, C.-C., Rubio-Cuadrado, Á., Sánchez-Salguero, R., Šamonil, P., Stajić, B., Svoboda, M., Tognetti, R., Toromani, E., Trotsiuk, V., van der Maaten, E., van der Maaten-Theunissen, M., Vannoppen, A., Vašíčková, I., von Arx, G., Wilmking, M., Weigel, R., Zlatanov, T., Zang, C., and Buras, A.: No Future Growth Enhancement Expected at the Northern Edge for European Beech due to Continued Water Limitation, Glob. Change Biol., 30, e17546, https://doi.org/10.1111/gcb.17546, 2024.
Körner, C.: Carbon limitation in trees, J. Ecol., 91, 4–17, https://doi.org/10.1046/j.1365-2745.2003.00742.x, 2003.
Körner, C.: Paradigm shift in plant growth control, Curr. Opin. Plant Biol., 25, 107–114, https://doi.org/10.1016/j.pbi.2015.05.003, 2015.
Laumer, W., Andreu, L., Helle, G., Schleser, G. H., Wieloch, T., and Wissel, H.: A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses, Rapid Commun. Mass Sp., 23, 1934–1940, https://doi.org/10.1002/rcm.4105, 2009.
Lefcheck, J. S.: PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics, Meth. Ecol. Evol., 7, 573–579, https://doi.org/10.1111/2041-210X.12512, 2016.
Lévesque, M., Siegwolf, R., Saurer, M., Eilmann, B., and Rigling, A.: Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions, New Phytol., 203, 94–109, https://doi.org/10.1111/nph.12772, 2014.
Liang, E., Leuschner, C., Dulamsuren, C., Wagner, B., and Hauck, M.: Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau, Climatic Change, 134, 163–176, https://doi.org/10.1007/s10584-015-1531-y, 2016a.
Liang, E., Wang, Y., Piao, S., Lu, X., Camarero, J. J., Zhu, H., Zhu, L., Ellison, A. M., Ciais, P., and Penuelas, J.: Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau, P. Natl. Acad. Sci. USA, 113, 4380–4385, https://doi.org/10.1073/pnas.1520582113, 2016b.
Linares, J. C. and Camarero, J. J.: From pattern to process: linking intrinsic water-use efficiency to drought-induced forest decline, Glob. Change Biol., 18, 1000–1015, https://doi.org/10.1111/j.1365-2486.2011.02566.x, 2012.
Lindner, M., Maroschek, M., Netherer, S., Kremer, A., Barbati, A., Garcia-Gonzalo, J., Seidl, R., Delzon, S., Corona, P., Kolstrom, M., Lexer, M. J., and Marchetti, M.: Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems, Forest Ecol. Manag., 259, 698–709, https://doi.org/10.1016/j.foreco.2009.09.023, 2010.
Liu, M., Trugman, A., Penuelas, J., and Anderegg, W.: Climate-driven disturbances amplify forest drought sensitivity, Nat. Clim. Chang., 14, 746–752, https://doi.org/10.1038/s41558-024-02022-1, 2024.
Liu, X., Zhao, L., Voelker, S., Xu, G., Zeng, X., Zhang, X., Zhang, L., Sun, W., Zhang, Q., Wu, G., and Li, X.: Warming and CO2 enrichment modified the ecophysiological responses of Dahurian larch and Mongolia pine during the past century in the permafrost of northeastern China, Tree Physiol., 39, 88–103, https://doi.org/10.1093/treephys/tpy060, 2019.
Loader, N. J., Robertson, I., Barker, A. C., Switsur, V. R., and Waterhouse, J. S.: An improved technique for the batch processing of small wholewood samples to alpha-cellulose, Chem. Geol., 136, 313–317, https://doi.org/10.1016/S0009-2541(96)00133-7, 1997.
Martínez-Sancho, E., Dorado-Linan, I., Merino, E. G., Matiu, M., Helle, G., Heinrich, I., and Menzel, A.: Increased water-use efficiency translates into contrasting growth patterns of Scots pine and sessile oak at their southern distribution limits, Glob. Change Biol., 24, 1012–1028, https://doi.org/10.1111/gcb.13937, 2018.
Martínez-Vilalta, J. and Garcia-Forner, N.: Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept, Plant Cell Environ., 40, 962–976, https://doi.org/10.1111/pce.12846, 2017.
McCarroll, D. and Loader, N. J.: Stable isotopes in tree rings, Q. Sci. Rev., 23, 771–801, https://doi.org/10.1016/j.quascirev.2003.06.017, 2004.
Mirabel, A., Girardin, M., Metsaranta, J., Way, D., and Reich, P.: Increasing atmospheric dryness reduces boreal forest tree growth, Nat. Commun., 14, 6901, https://doi.org/10.1038/s41467-023-42466-1, 2023.
Mu, Y. M., Fang, O., and Lyu, L.: Nighttime warming alleviates the incidence of juniper forest growth decline on the Tibetan Plateau, Sci. Total Environ., 782, 146924, https://doi.org/10.1016/j.scitotenv.2021.146924, 2021a.
Mu, Y. M., Zhang, Q. B., Fang, O. Y., Lyu, L. X., and Cherubini, P.: Pervasive tree-growth reduction in Tibetan juniper forests, Forest Ecol. Manag., 480, 118642, https://doi.org/10.1016/j.foreco.2020.118642, 2021b.
Nakagawa, S. and Schielzeth, H.: A general and simple method for obtaining R2 from generalized linear mixed-effects models, Meth. Ecol. Evol., 4, 133–142, https://doi.org/10.1111/j.2041-210x.2012.00261.x, 2013.
Olano, J., Sangüesa-Barreda, G., García-López, M., García-Hidalgo, M., Rozas, V., García-Cervigón, A., Delgado Huertas, A., and Hernández-Alonso, H.: Water use efficiency and climate legacies dominate beech growth at its rear edge, J. Ecol., 111, 2160–2171, https://doi.org/10.1111/1365-2745.14164, 2023.
Pan, Y. D., Birdsey, R. A., Phillips, O. L., Houghton, R. A., Fang, J. Y., Kauppi, P. E., Keith, H., Kurz, W. A., Ito, A., Lewis, S. L., Nabuurs, G. J., Shvidenko, A., Hashimoto, S., Lerink, B., Schepaschenko, D., Castanho, A., and Murdiyarso, D.: The enduring world forest carbon sink, Nature, 631, 563–569, https://doi.org/10.1038/s41586-024-07602-x, 2024.
Panthi, S., Fan, Z.-X., van der Sleen, P., and Zuidema, P. A.: Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate, Glob. Change Biol., 26, 1778–1794, https://doi.org/10.1111/gcb.14910, 2020.
Peñuelas, J., Canadell, J. G., and Ogaya, R.: Increased water-use efficiency during the 20th century did not translate into enhanced tree growth, Global Ecol. Biogeogr., 20, 597–608, https://doi.org/10.1111/j.1466-8238.2010.00608.x, 2011.
Peñuelas, J., Sardans, J., Estiarte, M., Ogaya, R., Carnicer, J., Coll, M., Barbeta, A., Rivas-Ubach, A., Llusia, J., Garbulsky, M., Filella, I., and Jump, A. S.: Evidence of current impact of climate change on life: a walk from genes to the biosphere, Glob. Change Biol., 19, 2303–2338, https://doi.org/10.1111/gcb.12143, 2013.
Peters, W., van der Velde, I. R., van Schaik, E., Miller, J. B., Ciais, P., Duarte, H. F., van der Laan-Luijkx, I. T., van der Molen, M. K., Scholze, M., Schaefer, K., Vidale, P. L., Verhoef, A., Warlind, D., Zhu, D., Tans, P. P., Vaughn, B., and White, J. W. C.: Increased water-use efficiency and reduced CO2 uptake by plants during droughts at a continental scale, Nat. Geosci., 11, 744–748, https://doi.org/10.1038/s41561-018-0212-7, 2018.
Piao, S., Nan, H., Huntingford, C., Ciais, P., Friedlingstein, P., Sitch, S., Peng, S., Ahlström, A., Canadell, J. G., Cong, N., Levis, S., Levy, P. E., Liu, L., Lomas, M. R., Mao, J., Myneni, R. B., Peylin, P., Poulter, B., Shi, X., Yin, G., Viovy, N., Wang, T., Wang, X., Zaehle, S., Zeng, N., Zeng, Z., and Chen, A.: Evidence for a weakening relationship between interannual temperature variability and northern vegetation activity, Nat. Commun., 5, 5018, https://doi.org/10.1038/ncomms6018, 2014.
Pu, X. and Lyu, L.: Disentangling the impact of photosynthesis and stomatal conductance on rising water-use efficiency at different altitudes on the Tibetan plateau, Agr. Forest Meteorol., 341, 109659, https://doi.org/10.1016/j.agrformet.2023.109659, 2023.
Pu, X., Wang, X., and Lyu, L.: Recent warming-induced tree growth enhancement at the Tibetan treeline and the link to improved water-use efficiency, Forests, 12, 1702, https://doi.org/10.3390/f12121702, 2021.
Qi, Z. H., Liu, H. Y., Wu, X. C., and Hao, Q.: Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, Northwestern China, Glob. Change Biol., 21, 816–826, https://doi.org/10.1111/gcb.12703, 2015.
R Core Team: R: a language and environment for statistical computing, R foundation for statistical computing, Vienna [software], https://www.r-project.org (last access: 29 November 2022), 2022.
Reed, C. C., Ballantyne, A. P., Cooper, L. A., and Sala, A.: Limited evidence for CO2-related growth enhancement in northern Rocky Mountain lodgepole pine populations across climate gradients, Glob. Change Biol., 24, 3922–3937, https://doi.org/10.1111/gcb.14165, 2018.
Salzer, M. W., Hughes, M. K., Bunn, A. G., and Kipfmueller, K. F.: Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes, Proc. Natl. Acad. Sci. U.S.A., 106, 20348–20353, https://doi.org/10.1073/pnas.0903029106, 2009.
Saurer, M., Siegwolf, R. T. W., and Schweingruber, F. H.: Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years, Glob. Change Biol., 10, 2109–2120, https://doi.org/10.1111/j.1365-2486.2004.00869.x, 2004.
Saurer, M., Spahni, R., Frank, D. C., Joos, F., Leuenberger, M., Loader, N. J., McCarroll, D., Gagen, M., Poulter, B., Siegwolf, R. T. W., Andreu-Hayles, L., Boettger, T., Linan, I. D., Fairchild, I. J., Friedrich, M., Gutierrez, E., Haupt, M., Hilasvuori, E., Heinrich, I., Helle, G., Grudd, H., Jalkanen, R., Levanic, T., Linderholm, H. W., Robertson, I., Sonninen, E., Treydte, K., Waterhouse, J. S., Woodley, E. J., Wynn, P. M., and Young, G. H. F.: Spatial variability and temporal trends in water-use efficiency of European forests, Glob. Change Biol., 20, 3700–3712, https://doi.org/10.1111/gcb.12717, 2014.
Shestakova, T. A., Voltas, J., Saurer, M., Berninger, F., Esper, J., Andreu-Hayles, L., Daux, V., Helle, G., Leuenberger, M., Loader, N. J., Masson-Delmotte, V., Saracino, A., Waterhouse, J. S., Schleser, G. H., Bednarz, Z., Boettger, T., Dorado-Linan, I., Filot, M., Frank, D., Grabner, M., Haupt, M., Hilasvuori, E., Jungner, H., Kalela-Brundin, M., Krapiec, M., Marah, H., Pawelczyk, S., Pazdur, A., Pierre, M., Planells, O., Pukiene, R., Reynolds-Henne, C. E., Rinne-Garmston, K. T., Rita, A., Sonninen, E., Stievenard, M., Switsur, V. R., Szychowska-Krapiec, E., Szymaszek, M., Todaro, L., Treydte, K., Vitas, A., Weigl, M., Wimmer, R., and Gutierrez, E.: Spatio-temporal patterns of tree growth as related to carbon isotope fractionation in European forests under changing climate, Global Ecol. Biogeogr., 28, 1295–1309, https://doi.org/10.1111/geb.12933, 2019.
Shi, C. M., Schneider, L., Hu, Y., Shen, M., Sun, C., Xia, J. Y., Forbes, B. C., Shi, P. L., Zhang, Y. D., and Ciais, P.: Warming-induced unprecedented high-elevation forest growth over the monsoonal Tibetan Plateau, Environ. Res. Lett., 15, 054011, https://doi.org/10.1088/1748-9326/ab7b9b, 2020.
Shipley, B.: Confirmatory path analysis in a generalized multilevel context, Ecology, 90, 363–368, https://doi.org/10.1890/08-1034.1, 2009.
Sigdel, S. R., Wang, Y. F., Camarero, J. J., Zhu, H. F., Liang, E. Y., and Penuelas, J.: Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas, Glob. Change Biol., 24, 5549–5559, https://doi.org/10.1111/gcb.14428, 2018.
Silva, L. C. R. and Anand, M.: Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes, Global Ecol. Biogeogr., 22, 83–92, https://doi.org/10.1111/j.1466-8238.2012.00783.x, 2013.
Silva, L. C. R., Sun, G., Zhu-Barker, X., Liang, Q. L., Wu, N., and Horwath, W. R.: Tree growth acceleration and expansion of alpine forests: The synergistic effect of atmospheric and edaphic change, Sci. Adv., 2, e1501302, https://doi.org/10.1126/sciadv.1501302, 2016.
Sterck, F., Song, Y., and Poorter, L.: Drought- and heat-induced mortality of conifer trees is explained by leaf and growth legacies, Sci. Adv., 10, eadl4800, https://doi.org/10.1126/sciadv.adl4800, 2024.
van der Sleen, P., Groenendijk, P., Vlam, M., Anten, N. P. R., Boom, A., Bongers, F., Pons, T. L., Terburg, G., and Zuidema, P. A.: No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased, Nat. Geosci., 8, 24–28, https://doi.org/10.1038/Ngeo2313, 2015.
Voelker, S. L., Brooks, J. R., Meinzer, F. C., Anderson, R., Bader, M. K. F., Battipaglia, G., Becklin, K. M., Beerling, D., Bert, D., Betancourt, J. L., Dawson, T. E., Domec, J. C., Guyette, R. P., Korner, C., Leavitt, S. W., Linder, S., Marshall, J. D., Mildner, M., Ogee, J., Panyushkina, I., Plumpton, H. J., Pregitzer, K. S., Saurer, M., Smith, A. R., Siegwolf, R. T. W., Stambaugh, M. C., Talhelm, A. F., Tardif, J. C., Van de Water, P. K., Ward, J. K., and Wingate, L.: A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: evidence from carbon isotope discrimination in paleo and CO2 enrichment studies, Glob. Change Biol., 22, 889–902, https://doi.org/10.1111/gcb.13102, 2016.
Voltas, J., Aguilera, M., Gutiérrez, E., and Shestakova, T. A.: Shared drought responses among conifer species in the middle Siberian taiga are uncoupled from their contrasting water-use efficiency trajectories, Sci. Total Environ., 720, 137590, https://doi.org/10.1016/j.scitotenv.2020.137590, 2020.
Walker, X. J., Mack, M. C., and Johnstone, J. F.: Stable carbon isotope analysis reveals widespread drought stress in boreal black spruce forests, Glob. Change Biol., 21, 3102–3113, https://doi.org/10.1111/gcb.12893, 2015.
Wang, B., Chen, T., Xu, G. B., Wu, M. H., Zhang, G. S., Li, C. J., and Wu, G. J.: Anthropogenic-management could mitigate declines in growth and survival of Qinghai spruce (Picea crassifolia) in the east Qilian Mountains, northeast Tibetan Plateau, Agr. Forest Meteorol., 250, 118–126, https://doi.org/10.1016/j.agrformet.2017.12.249, 2018.
Wang, J., Taylor, A., and D'Orangeville, L.: Warming-induced tree growth may help offset increasing disturbance across the Canadian boreal forest, P. Natl. Acad. Sci. USA, 120, e2212780120, https://doi.org/10.1073/pnas.2212780120, 2023.
Wang, W., McDowell, N. G., Liu, X., Xu, G., Wu, G., Zeng, X., and Wang, G.: Contrasting growth responses of Qilian juniper (Sabina przewalskii) and Qinghai spruce (Picea crassifolia) to CO2 fertilization despite common water-use efficiency increases at the northeastern Qinghai-Tibetan Plateau, Tree Physiol., https://doi.org/10.1093/treephys/tpaa169, 2020.
Wang, W. Z., Liu, X. H., An, W. L., Xu, G. B., and Zeng, X. M.: Increased intrinsic water-use efficiency during a period with persistent decreased tree radial growth in northwestern China: Causes and implications, Forest Ecol. Manag., 275, 14–22, https://doi.org/10.1016/j.foreco.2012.02.027, 2012.
Wu, G. J., Liu, X. H., Chen, T., Xu, G. B., Wang, W. Z., Zeng, X. M., and Zhang, X. W.: Elevation-dependent variations of tree growth and intrinsic water-use efficiency in Schrenk spruce (Picea schrenkiana) in the western Tianshan Mountains, China, Front. Plant Sci., 6, 12, https://doi.org/10.3389/fpls.2015.00309, 2015.
Xu, G. B., Liu, X. H., Qin, D. H., Chen, T., An, W. L., Wang, W. Z., Wu, G. J., Zeng, X. M., and Ren, J. W.: Climate warming and increasing atmospheric CO2 have contributed to increased intrinsic water-use efficiency on the northeastern Tibetan Plateau since 1850, Trees-Struct. Funct., 27, 465–475, https://doi.org/10.1007/s00468-013-0855-3, 2013.
Yang, R.-Q., Fu, P.-L., Fan, Z.-X., Panthi, S., Gao, J., Niu, Y., Li, Z.-S., and Bräuning, A.: Growth-climate sensitivity of two pine species shows species-specific changes along temperature and moisture gradients in southwest China, Agr. Forest Meteorol., 318, 108907, https://doi.org/10.1016/j.agrformet.2022.108907, 2022.
Zhang, X., Liu, X., Zhang, Q., Zeng, X., Xu, G., Wu, G., and Wang, W.: Species-specific tree growth and intrinsic water-use efficiency of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) growing in a boreal permafrost region of the Greater Hinggan Mountains, Northeastern China, Agr. Forest Meteorol., 248, 145–155, https://doi.org/10.1016/j.agrformet.2017.09.013, 2018.
Zhao, D., Zhang, Z., and Zhang, Y.: Soil Moisture Dominates the Forest Productivity Decline During the 2022 China Compound Drought-Heatwave Event, Geophys. Res. Lett., 50, e2023GL104539, https://doi.org/10.1029/2023GL104539, 2023.




