the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reviews and syntheses: Artisanal and small-scale gold mining (ASGM)-derived mercury contamination in agricultural systems: what we know and need to know
David S. McLagan
Excellent O. Eboigbe
Rachel J. Strickman
The escalating global demand for gold has fuelled the rapid expansion of artisanal and small-scale gold mining (ASGM), which has become the largest source of mercury (Hg) emissions worldwide. Here we synthesize current research on the pervasive contamination of agricultural systems by ASGM-derived Hg, identifying the key environmental pathways and subsequent risks to food security. Within these systems, Hg undergoes complex biogeochemical transformations, with the methylation of inorganic Hg into its highly neurotoxic form, methylmercury (MeHg), being a critical process. This is particularly pronounced in rice paddy systems, where microbial activity and favourable redox conditions facilitate Hg methylation, resulting in the bioaccumulation of MeHg in rice grains – a staple food for billions. However, this synthesis reveals that atmospheric uptake is important to total Hg loadings in rice, and more so in tissues of crops grown in unsaturated soils. Indeed, we stress the importance of assessing all potential uptake pathways of Hg in agricultural systems: foliar assimilation from air, uptake from soils/water (particularly MeHg in rice), direct deposition to surfaces, and consumption of contaminated crop tissues (by both humans and livestock/poultry), to delineate the source and ratios of the different pools of Hg within crops and their consumers. A common shortcoming in past studies of ASGM-derived Hg in agricultural systems is that they have commonly overlooked one or more of these uptake pathways. These findings underscore a significant threat to global food chains and human health through the consumption of Hg contaminated produce. Mitigating these risks requires an improved understanding of the quantity of emissions/releases from ASGM, input pathways, and Hg biogeochemical cycling and fate in agricultural landscapes, paving the way for targeted interventions and sustainable management strategies to protect vulnerable communities. We suggest that these goals can be achieved through strategic international and interdisciplinary collaborations, novel and accessible technologies, and care for the dissemination of scientific information to impacted communities.
As a transition metal with distinctive physicochemical properties, including unique relativistic effects, high surface tension, and liquid state at ambient temperature and pressure, mercury (Hg) is a unique and environmentally significant element (Norrby, 1991; Jasinski, 1995; Fitzgerald and Lamborg, 2005). These unique properties have captivated many civilizations throughout history, with Hg being used across a range of applications including paint pigmentation, medicinal, and spiritual ceremonies (Bagley et al., 1987; Hardy et al., 1995; Jiang et al., 2006). Use of Hg continues into the modern era particularly in industrial, mining, and medical applications (Finster et al., 2015; Munthe et al., 2019). Hg's recognition as a global pollutant relates to its environmental persistence, long-range transport capabilities, and negative impacts on human and environmental health (i.e., neurotoxicity) (Durnford et al., 2010; Driscoll et al., 2013; Fitzgerald et al., 2007).
While all forms of Hg are toxic and we are yet to discover a biological function of the element in the Eukarya domain at least (Peralta-Videa et al., 2009; Cozzolino et al., 2016; Grégoire and Poulain, 2016), methyl-Hg (MeHg) is the most toxic and bioaccumulative form and the source of the majority of Hg's impacts on human and environmental health (Clarkson et al., 2003; Bjørklund et al., 2017). The effects of Hg (and particularly MeHg) exposure on children, both in utero and after birth, are of particular concern due to Hg's primary toxicological action being neurological, causing abnormalities during foetal development, neurodevelopmental delays during childhood, with connections to autism and other mental disabilities (Schettler, 2001; Bose-O'Reilly et al., 2010; Kern et al., 2016; dos Santos-Lima et al., 2020). There are also links between Hg exposure and adverse effects on cardiovascular, gastrointestinal, renal (kidneys), and pulmonary systems (Ha et al., 2017; Basu et al., 2023).
In 2013, a global treaty on Hg, the Minamata Convention, was brought into effect and signed by 128 nations (UNEP, 2013), with the primary goal of reducing the impacts of Hg on human and environmental health. The texts and annexes of the Minamata Convention lay out the scientific and policy means to achieve these goals including a focus on decreasing levels of Hg emitted to the atmosphere and released to land, water and oceans, from activities such as artisanal and small-scale gold mining (ASGM) by promoting more sustainable gold mining practices and controlling the supply and trade of Hg (UNEP, 2013).
1.1 The biogeochemical cycle of mercury
Hg can exist in various oxidation states in the environment. This includes Hg(0) (elemental or metallic), divalent or mercuric, and Hg(I) (monovalent/mercurous), although the latter is uncommon and highly unstable in the environment and is rather a short-lived intermediary between Hg(0) and divalent Hg (Schuster, 1991; Schroeder and Munthe, 1998). Hg(0) dominates the atmosphere, inorganic divalent Hg (IHg(II)1) is the predominant form in water, soil, and sediments, and MeHg (organic divalent Hg) is the dominant form in biota (Guzzi and La Porta, 2008; Ullrich et al., 2001; Fitzgerald et al., 2007). IHg(II) compounds are numerous and exhibit distinct physicochemical properties (i.e., HgCl2 is highly soluble, while HgS, or cinnabar, is practically insoluble) that govern their behaviour and cycling in the environment (Schroeder and Munthe, 1998; Ulrich et al., 2001; Clarkson and Magos, 2006; Park and Zheng, 2012; Barkay and Wagner-Döbler, 2005). While Hg is found in a wide range of minerals, the most abundant Hg-containing minerals are cinnabar (α-HgS) and metacinnabar (β-HgS) (Nöller, 2014).
The global distribution of Hg is achieved primarily through the atmosphere as Hg(0) (Lindberg et al., 2007; Gworek et al., 2020), driven by its high volatility and low solubility (Henry's law constant: Pa−1; Andersson et al., 2008; Gaffney and Marley, 2014), which results in a long atmospheric lifetime of ≈4–18 months (Holmes et al., 2010; Horowitz et al., 2017; Saiz-Lopez et al., 2018). Long-range transport via river systems also contributes, although it is less important than the atmospheric transport pathway (Ariya et al., 2015; Dastoor et al., 2022). Removal from the atmosphere occurs via dry deposition of Hg(0) (dominant pathway in terrestrial systems; see Sect. 3 below) or oxidation to gas- or particulate-phase IHg(II) and subsequent wet and dry deposition of these less volatile forms (Ariya et al., 2015; Zhou et al., 2021; Dastoor et al., 2025). These depositional processes to terrestrial and aquatic systems represent exchanges (negative fluxes), and the reverse processes (including reduction of IHg(II) back to Hg(0) and subsequent volatilization; positive fluxes) can also occur (Outridge et al., 2018; Dastoor et al., 2025). It is only through burial in sedimentary materials (ocean sediments, lake sediments, and subsurface soils) that Hg is removed from the active biogeochemical cycle (Fitzgerald and Lamborg, 2005; Outridge et al., 2018).
IHg(II) compounds deposited, produced in situ from Hg(0) oxidation, emitted directly as IHg(II) to air from some industrial source, or released directly into aquatic environments such as wetlands, rivers, and lakes can undergo microbially mediated (both enzymatic and non-enzymatic) processes that catalyse the transfer of methyl groups from donors like methylcobalamin to IHg(II) species, forming MeHg compounds (Ullrich et al., 2001). Methylation typically occurs under anoxic conditions in saturated sediments and soils, but some recent studies suggest that methylation could also proceed under oxic conditions in certain scenarios (Gallorini and Loizeau, 2021; Wang et al., 2021b). Representatives of sulphur-reducing bacteria, iron-reducing bacteria, methanogens, diverse firmicutes, and other fermenting bacteria have been identified to predominantly mediate this process in the environment (Compeau and Bartha, 1985; Lei et al., 2023). The produced MeHg readily binds to organic matter (OM; in sediments/particles), can be taken up by consumers, bioaccumulated, and then biomagnified up food webs (Ariya et al., 2015). MeHg can also be demethylated biotically and abiotically (Kritee et al., 2007; Barkay and Gu, 2021). Biotic demethylation has been posited to proceeds via two pathways: (i) reductive or mer-dependent demethylation (taxonomically widely distributed, and common in more contaminated environments) and (ii) oxidative or mer-independent demethylation (less well understood) (Barkay and Gu, 2021). Abiotic demethylation occurs via direct or indirect photolysis (Barkay and Gu, 2021).
Study of the Hg biochemical cycle has advanced significantly in the past two decades since the development of cold-vapour introduction methods for multi-collector, inductively-coupled plasma, mass spectrometers (MCICPMS) that has facilitated high precision measurement and analyses of natural abundance Hg stable isotopes in samples spanning a broad range of environmental matrices (Bergquist and Blum, 2009). There are seven stable isotopes of Hg and significant mass-dependent (MDF; defined by δ notation) and mass-independent fractionation (MIF; defined by Δ notation) have been observed across a broad range of natural and anthropogenically driven processes and reactions (Bergquist and Blum, 2009; Sun et al., 2019; Tsui et al., 2020). Tracking Hg sources and processes with stable isotopes analyses across time and space transcends conventional concentration analyses by providing unique insights into the intricate behaviour and transformations of Hg across diverse ecosystems at local, regional and global scales (Bergquist and Blum, 2009; Sun et al., 2019; Tsui et al., 2020). Studies applying Hg spikes of enriched tracer isotopes (typically in lab or heavily controlled field mesocosm experiments) have been frequently used within the literature and are largely based on the same theoretical principles used in natural abundance stable isotope analyses but can exploit less robust/precise instrumentation (i.e., quadrupole ICPMS) due to the applied artificial isotope enrichments (Hintelmann et al., 2000; Strickman and Mitchell, 2017).
1.2 Sources of mercury to the environment
It is important to distinguish primary emissions of Hg (predominantly to air) that augment the mass of Hg within the active biogeochemical cycle from reemissions that represent positive fluxes of Hg from terrestrial and aquatic matrices (i.e., vegetation, soils, water bodies) to air, but do not alter the actively cycling mass of Hg. Reemissions more appropriately characterize processes such as biomass burning (including wildfires) and land use change that drive Hg back to the atmosphere as exchange process (be they anthropogenically driven or not) rather than emissions sources (Outridge et al., 2018; Dastoor et al., 2025). Hence, the focus of this section will be on the primary sources of Hg emissions.
Natural primary emissions of Hg (geogenic activities and weathering of Hg-containing rocks) are estimated at 76–300 Mg yr−1 and make up a minor component of total annual emissions from primary sources (Streets et al., 2019, and references therein). The most recent inventories of primary anthropogenic emissions of Hg to air are from 2015 by Streets et al. (2019) and Munthe et al. (2019); these sources estimate annual emissions to be 2390 (+42 %/−19 %) Mg yr−1 and 2220 (+27 %/−10 %) Mg yr−1, respectively. In addition, Munthe et al. (2019), estimated 583 Mg yr−1 (nonspecific uncertainty; described as large for this estimate) of Hg are released to aquatic systems2.
Changing anthropogenic sources
Historically, the combustion of fossil fuels (particularly coal) has been considered the largest anthropogenic source of mercury emissions globally (Pacyna et al., 2006, 2010; Pirrone et al., 2010; Streets et al., 2011). The high temperatures achieved during fossil fuel combustion liberate any residual Hg and release it as Hg(0), which typically undergoes partial oxidation after combustion to gaseous and particulate-bound divalent Hg forms (Carpi, 1997; Pacyna et al., 2006). More recent assessments indicate that ASGM (defined in Sect. 2) is now the largest global source of anthropogenically derived Hg (Streets et al., 2019; Munthe et al., 2019; Yoshimura et al., 2021). Munthe et al. (2019) estimate the total ASGM emissions of Hg to air to be 838 ± 163 Mg yr−1 (37.7 % of total global Hg emissions to air) and total ASGM releases of Hg to water and land to be 1221 (±637) Mg yr−1. However, the authors caution that the ASGM estimate represents a highly uncertain, “special” case scenario due to the challenges in estimating emissions/releases from a sector with such large knowledge gaps (Munthe et al., 2019); therefore, even these large uncertainty ranges may be underestimates. Most ASGM Hg emissions estimates rely on a bottom-up approach based on gold production and emission factors rather than actual Hg use (Pfeiffer and Lacerda, 1988; Seccatore et al., 2014; Streets et al., 2019; Munthe et al., 2019; Yoshimura et al., 2021). Moreover, there is large variability not only between estimates made by different groups, but also between different regions where ASGM occurs (Seccatore et al., 2014; Yoshimura et al., 2021). The informal and often illegal nature of ASGM activities, which have grown rapidly in recent decades (Wagner and Hunter, 2020; Bernet Kempers, 2020; see also Sect. 2), present major challenges to Hg use inventorying (Hilson, 2008; Veiga and Marshall, 2019).
Hentschel et al. (2002) of the International Institute for Environment and Development (IIED) define artisanal and small-scale mining as “mining by individuals, groups, families or cooperatives with minimal or no mechanisation, often in the informal (illegal) sector of the market”. However, the IIED (and many other organizations and researchers) stress that a formal definition is still lacking, and an increasing degree of mechanization and larger scale operations are defined under artisanal and small-scale mining in many jurisdictions (Hentschel et al., 2002). This review focusses on gold mining (ASGM) alone due to the unique use of Hg in the gold extraction process.
ASGM encompasses a wide range of techniques used to extract gold and activities range from legal and regulated to informal to illegal activities (Veiga et al., 2006) and it contributes ≈20 %–30 % of the world's gold production (Swain et al., 2007; Telmer and Veiga, 2009). Estimates suggest ≈20 million individuals (including ≈3 million women and children) across >70 countries (mainly in Africa, Asia, and South and Central America) are directly engaged in ASGM (Seccatore et al., 2014; UNEP, 2017, Veiga and Gunson, 2020). Participant numbers increase to at least 100 million when people indirectly dependent upon ASGM for their livelihood are also considered (Telmer and Veiga, 2009; Veiga and Baker, 2004). The (near) exponential growth of the ASGM sector in recent years can be attributed to soaring gold prices, and the ease of entry into the sector and selling gold (Veiga and Hinton, 2002; Adranyi et al., 2023). For example, the world gold spot price has increased by an order of magnitude from ≈ USD 9000 kg−1 in 2000 to ≈ USD 125 000 kg−1 as of 2025 (World Gold Council, 2025). For many miners, particularly those in rural communities in the Global South, employment and survival serve as primary motivators and ASGM offers substantial financial rewards during peak periods (Teschner, 2014; Wilson et al., 2015; Tschakert, 2009). However, Adranyi et al. (2023) argue that these benefits come at significant social costs, which include impacts on alternative livelihoods (i.e., loss of income for farmers as ASGM encroaches on agricultural areas, which turns many individuals to ASGM).
The profitability of ASGM, legislative restrictions on the sector, and its proclivity to be practiced in remote areas with less police/military presence combine to foster an environment conducive to criminal activities led by local gangs, domestic and transnational organized crime syndicates, and illegal armed groups (Diaz et al., 2020; Schwartz et al., 2021). Bugmann et al. (2022) explain how industry forces are exploiting market opportunities and coercing individuals into mining labour. Nevertheless, neither the (il)legality nor the awareness of ASGM's impacts on human and environmental health (albeit often limited awareness; Osei et al., 2022) have had much impact on the popularity of ASGM or the use of Hg in the gold extraction and refinement processes (Veiga et al., 2006; Veiga and Gunson, 2020; Thomas et al., 2019). The allure of substantial financial gains, the scarcity of viable alternatives, and the lack of incentives for sustainable practices all contribute to the complexity of reform within this sector (Veiga and Gunson, 2020; Telmer and Veiga, 2009).
The ASGM Hg amalgamation process and its impacts
Hg is used to extract gold directly from the entire mined ore (less efficient: 10–25 g of Hg per gram of gold) or from gravity ore concentrate (gold-enriched heavy fraction; more efficient: 1–3 g of Hg per gram of gold) by exploiting the natural solid amalgam that forms when gold and Hg(0) come in contact (Veiga et al., 1995, 2014; Yoshimura et al., 2021). This process produces the solid Hg-gold amalgam, tailings (waste), and residual liquid Hg, the latter of which is reused a few times until it becomes less effective and “dirty” (inefficient), at which point it is typically discarded into the environment (Telmer and Veiga, 2009). Once the Hg-gold amalgam is formed (typically ≈60 % gold by mass), subsequent gold extraction is typically accomplished by roasting of amalgam using rudimentary setups in open air, which results in volatilization of Hg directly into the atmosphere while leaving the gold behind (Veiga and Hinton, 2002; Kiefer et al., 2015; Ogola et al., 2002). This gold contains ≈2 %–5 % residual Hg (Veiga and Hinton, 2002) and is typically roasted a second time after purchasing by initial gold traders (Cordy et al., 2011, 2013; Moody et al., 2020; Veiga et al., 2014). Although retorts allow near complete recovery of Hg during amalgam burning, their uptake and widespread use are limited due to costs, lack of training, and other social issues (i.e., desire to visually observe the amalgam burning process) that are well-detailed in literature (Hinton et al., 2003; Hilson, 2006; Jønsson et al., 2013).
Alternatives to the Hg amalgamation process do exist. These include dissolution of Hg with nitric acid (Moreno-Brush et al., 2020; Cho et al., 2020) or the use of cyanide in place of Hg (Marshall et al., 2020). Yet these are not popular methods due to their own inherent social, financial, and environmental constraints (Telmer and Veiga, 2009; Brüger et al., 2018). In addition, cyanidation is used in parallel with Hg amalgamation both to improve gold extraction efficiencies and during transition away from Hg amalgamation (Malone et al., 2023; da Silva and Guimarães, 2024). Concurrent use of these two methods can lead to synergistic environmental and human health impacts as Hg-cyanide complexes are highly toxic and increase the solubility, and hence mobility, of Hg in ASGM wastes and tailings (Seney et al., 2020; da Silva and Guimarães, 2024). Hg amalgamation remains the preferred method employed by ASGM to extract gold due to its simplicity, efficiency, low cost, availability, and, ultimately, a greater confidence and trust in the Hg amalgamation process by miners. This latter point is emphasized by the aptly titled study by Bugmann et al. (2022): “Doing ASGM without mercury is like trying to make omelettes without eggs”: Understanding the persistence of mercury use among artisanal gold miners in Burkina Faso.
While emissions of Hg to air from ASGM activities can undergo long-range transport and contribute to Hg's global impacts, much is deposited locally or regionally (Munthe et al., 2019; Szponar et al., 2025). In addition, most direct releases of Hg from ASGM to terrestrial and aquatic systems are localised (Munthe et al., 2019; Moreno-Brush et al., 2020). Hence, communities living and working in proximity to ASGM areas are those that suffer the greatest health impacts from this activity including the miners who can experience both inhalation and direct dermal exposures when handling Hg(0) for gold extraction or burning amalgams (Veiga and Baker, 2004; Bose-O'Reilly et al., 2010; Taux et al., 2022).
Another common pathway of exposure is through the ingestion of organic Hg (i.e., MeHg) from dietary sources (Zahir et al., 2005). Fish, for instance, are exposed to MeHg both through their environment (water) and food, with diet accounting for approximately 80 %–90 % of their total intake (Zahir et al., 2005). This is of particular concern for communities impacted by ASGM activities whose major source of protein is fish (Vieira, 2006). Logically, research on dietary exposures to Hg in ASGM affected areas is dominated by fish-focussed studies; there are many examples of elevated concentrations of THg and/or MeHg in fish sampled in close proximity to ASGM activities (e.g., Barocas et al., 2023; Castilhos et al., 2015; Bose-O'Reilly et al., 2016; Maurice-Bourgoin et al., 1999). Nonetheless, fish is not the only food consumed in regions impacted by ASGM activities.
The surface and/or near-surface mining activities that dominate ASGM are major drivers of land-cover change. ASGM accounts for ≈7 % of deforestation in the Global South (Hosonuma et al., 2012; Timsina et al., 2022). Additionally, the recovery of forests after mining activities is slower when compared to other land uses (Timsina et al., 2022). ASGM increases particle loading to rivers caused by erosion directly from ASGM activities or indirectly after deforestation (Swenson et al., 2011; Esdaile and Chalker, 2018; Moreno-Brush et al., 2020). These issues of mining-driven deforestation and increased riverine sediment loadings present major environmental health issues in their own rights and are the focus of many other studies and reviews (e.g., Moreno-Brush et al., 2020; Timsina et al., 2022; Dossou Etui et al., 2024). In addition, anthropogenically modified land-covers such as lands used for agriculture are increasingly finding themselves in direct competition for space with ASGM (Achina-Obeng and Aram, 2022; Adranyi et al., 2023; Yu et al., 2024; Donkor et al., 2024). In Ghana, Achina-Obeng and Aram (2022) report that most lands converted from agriculture to ASGM are obtained from legal sales. However, contrary reports of ASGM “land-grabbing” also exist in Ghana and elsewhere (Gilbert and Albert, 2016; Malone et al., 2021; Adranyi et al., 2024). Indeed, conflicts between miners and farmers/farming communities (including Indigenous Peoples) are frequent (Mestanza-Ramón et al., 2022; Adranyi et al., 2024). A common conflict arises from the land, water and soil degradation inflicted by ASGM that typically renders previously arable lands to be less productive or simply infertile post mining (Gilbert and Albert, 2016; Adranyi et al., 2024). In many areas, ASGM and agriculture continue to operate alongside each other. A number of studies cite ASGM and Hg amalgam processing occurring directly adjacent to croplands, and farmers subsidizing their agricultural livelihood as part-time artisanal miners (Krisnayanti et al., 2012; Mestanza-Ramón et al., 2022; Adranyi et al., 2023, 2024; Adator et al., 2023). Hence, consumption of crops and livestock/poultry contaminated by ASGM-derived Hg presents an additional and much less explored potential pathway of human dietary Hg exposure (Xia et al., 2020; Sanga et al., 2023).
There are three potential pathways of Hg uptake in higher or vascular plants (the majority of food, feed, and fuel crops are derived from vascular plants): (1) stomatal assimilation of gas-phase Hg (0) during photosynthetic respiration, (2) surface sorption to cuticular (foliage) or periderm (stems/bole/edible tissues) surfaces, and (3) uptake from roots (Zhou et al., 2021; Liu et al., 2022; McLagan et al., 2022a); these processes are summarized in Fig. 1. Of these three pathways, stomatal assimilation is now considered to be the dominant mechanism and reported to be responsible for >90 % of all Hg found not only in foliage, but all above ground plant tissues (Beauford et al., 1977; Graydon et al., 2009; Rutter et al., 2011a, b; Laacouri et al., 2013; Zhou et al., 2021; Zhou and Obrist, 2021). Moreover, many crops are also utilized as feed for livestock and poultry. If these feedstocks are contaminated by Hg, there is potential for accumulation in livestock/poultry and transfer to humans after meat or animal by-product consumption. Within this section we will explore each of these exposure mechanisms as they relate to Hg derived from ASGM and discuss their relevancy and potential impacts on human health.
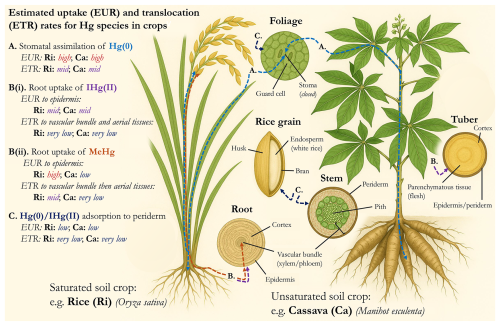
Figure 1Conceptual model summarizing the uptake and translocation processes of different Hg species in both saturated (i.e., rice) and unsaturated (i.e., cassava) soil crops including estimated qualitative rates based on the reviewed literature in Sect. 3.1 and 3.2. Line colours are associated with colours of species listed on the left (i.e., Hg(0) is in light blue). We note that plant and plant tissue art was developed for the purpose of presentation and is based on generic representations; hence, they may differ slightly from reality. Plant and plant tissue images were developed using ChatGPT (OpenAI), but all other parts of the figure (including labels) were constructed by co-authors.
3.1 Hg uptake in crops from air: the breathers
3.1.1 Atmospheric Hg uptake in higher plants
Research on the uptake mechanisms of Hg from air to vegetation is highly contemporary but contains many uncertainties and knowledge gaps. The surficial sorption pathway of Hg integration into internal foliar tissue is limited largely due to the potential for Hg sorbed to the foliar cuticle to be washed off by precipitation (Rea et al., 2000; Rutter et al., 2011a, b; Laacouri et al., 2013) or undergo photoreduction to Hg(0) and subsequently volatilize (Mowat et al., 2011; Laacouri et al., 2013). Dark/night experiments (when stomata are closed) have provided mixed results: some studies suggest a negative flux of Hg(0) to vegetation may occur (Converse et al., 2010; Fu et al., 2016), while other studies are less conclusive (Fritsche et al., 2008) or indicate strong correlations between Hg(0) uptake and stomatal conductance rates (higher uptake when stomata are open; Naharro et al., 2020; Denzler et al., 2025). While this suggests that a small fraction of gas-phase or surficially sorbed Hg(0) could diffuse through the cuticle and into the internal mesophyll, this diffusion-based process is mechanistically similar to stomatal uptake and would likely induce a similarly large, negative (favouring lighter isotopes) fractionation of Hg stable isotopes. As such, the discussion on atmospheric uptake pathways will focus on the stomatal assimilation mechanism and assume all Hg within the above ground parts of plants is derived from this uptake mechanism unless explicitly stated otherwise.
Stomatal assimilation has been directly linked to photosynthetic activity (net primary productivity; NPP) and consequently plant growth rates (Jiskra et al., 2018; Fu et al., 2019; Szponar et al., 2023). As such, stomatal assimilation by vegetation has been described as a global Hg(0) pump and accounts for the largest negative flux of Hg from air to terrestrial systems (Jiskra et al., 2018). Other factors such as stomatal conductance (itself impacted by atmospheric/meteorological/hydrological conditions), stomatal density, photosynthetic mechanism (i.e., C3 vs. C4), cuticle thickness, cuticle roughness, plant species, and plant and foliage life stages also influence Hg(0) uptake (Converse et al., 2010; Laacouri et al., 2013; Wohlgemuth et al., 2020, 2022; Liu et al., 2022; Eboigbe et al., 2025). In addition, the rate of Hg(0) foliar uptake, and consequently the THg concentration in foliage, is directly proportional to Hg(0) concentration in air (Navrátil et al., 2017; Manceau et al., 2018; Zhou et al., 2021), which makes the stomatal assimilation method particularly relevant in areas with substantial Hg(0) emissions to air, including ASGM regions. Confirmation of the dominance of the stomatal assimilation pathway and links to NPP (and other factors) has come largely within the last 10–15 years and owes much to advancements in Hg stable isotope research. Stomatal assimilation favours lighter isotopes and results in a MDF and shifts in δ202Hg values of between −1 ‰ and −3 ‰ compared to gas-phase Hg(0) (Zhou et al., 2021, and references therein), which creates an effective (light isotope) tracer for Hg uptake via this mechanism in plants.
After uptake of Hg(0) into internal foliar tissue, our understanding of the processes controlling the internal biogeochemical cycling within plants becomes somewhat less certain. Since foliar THg concentrations increase across the growing season (Rea et al., 2002; Laacouri et al., 2013; Wohlgemuth et al., 2020, 2022), Hg(0) must undergo oxidation to IHg(II) (Laacouri et al., 2013; Manceau et al., 2018) to maintain the high (air) to low (within foliage) Hg(0) concentration gradient that drives diffusion of Hg(0) into foliage. Limitations in the interpretive power of Hg speciation analysis (McLagan et al., 2022b) restrict our knowledge of the compounds responsible for this oxidation step, particularly at ambient concentrations. Nonetheless, Du and Fang (1983) linked foliar Hg uptake rates to enzymatic (catalase) activity in a high-concentration labelled isotope study, and studies using X-ray absorption techniques on foliage samples from plants growing under highly contaminated settings have identified Hg-thiol complexes and sulphur nanoparticles (Carrasco-Gil et al., 2013; Manceau et al., 2018) within foliage. We require more knowledge of the biological compounds responsible for oxidation and the resulting IHg(II) species, particularly as this could provide critical insight into the use of vegetation in contaminated site remediation such as at ASGM impacted areas.
As discussed, the stomatal assimilation pathway represents a net negative flux (Hg accumulation in vegetation) overall. However, re-release of Hg(0) taken up by this pathway has been posited to occur via photochemically-driven reduction of IHg(II) back to Hg(0) and release back out of the stomata. Using a Hg stable isotope mass balance model, Yuan et al. (2018) estimated that ≈30 % of assimilated Hg(0) is re-released from their studied species.
3.1.2 Translocation of Hg from foliage in higher plants
Assessments of the distribution of Hg across different plant tissues consistently indicate foliage has the highest THg concentrations (Zhou et al., 2017, 2021; Liu et al., 2021b). This accumulation in foliage (driven by stomatal assimilation) results in litterfall representing the major flux of Hg to soils in vegetated ecosystems (≈1000–1500 Mg yr−1) and these same estimates have typically also been used for as a proxy for net Hg assimilation flux into vegetation (Wang et al., 2016; Jiskra et al., 2018; Zhou et al., 2021). Yet it has been suggested that the use of litterfall alone likely results in a substantial underestimation of the net Hg vegetation assimilation flux due to the translocation of Hg from foliage into other tissues (i.e., branches, stems/boles, roots, seeds, flowers) (Zhou and Obrist, 2021). Indeed, despite bole wood having the lowest THg concentrations of any tree tissues (Zhou et al., 2017, 2021; Liu et al., 2021b), they contain the largest pool of Hg by mass of any tree tissues due to the much greater total biomass of bole wood compared to other tissues (Liu et al., 2021b). Hg storage in bole wood highlights the capacity of vegetation to translocate assimilated Hg away from foliage.
Phloem, vascular tissue that transports solutes (i.e., nutrients, proteins, and photosynthetic by-products such as sugars) away from the foliage within phloem sap, is suggested to be responsible for the downward translocation of Hg (Siwik et al., 2010; Zhou et al., 2021; Gačnik and Gustin, 2023). Throughout this downward migration, lateral translocation of Hg from phloem, through the cambium, and into the hydroactive xylem (sapwood) must occur. Evidence for this process lies in dendrochronological studies that (species/genus dependent) effectively archive historical Hg(0) concentrations in tree rings (e.g., Siwik et al., 2010; Navrátil et al., 2017; McLagan et al., 2022a; Gačnik and Gustin, 2023). Yanai et al. (2020) and Liu et al. (2024) went further and demonstrated that this translocation from phloem to xylem slowly reduces the amount of Hg within the phloem sap by observing a decrease in THg concentrations in tree rings of the same age from the canopy to the ground.
Liu et al. (2021b) and McLagan et al. (2022a) analysed tree bark for Hg stable isotopes, and data were highly negative in MDF (δ202Hg) and similar to xylem samples (tree rings) and foliage (in the case of Liu et al., 2021b). This indicates foliar uptake, phloem transport, and lateral translocation to periderm or cork (outer bark) is likely an important source of Hg in bark (we would expect more positive MDF associated with direct deposition from air as any such Hg would not be negatively fractionated during foliar uptake; Liu et al., 2021b; McLagan et al., 2022a). From our search there have been no studies in the literature assessing this theory in annual or bi-annual plants, such as agricultural crops.
Belowground tissues have received less attention than aboveground tissues, but Hg stable isotope data (negative δ202Hg values) from trees and shrubs in a high altitude forest in China indicated that 44 %–83 % of Hg in roots is derived from the stomatal assimilation pathway (Wang et al., 2020). Such data suggest root Hg storage and/or that plants could potentially detoxify by releasing Hg taken up from air into soils. Contrary to this, isotope data from wetland plants (i.e., rice) reflect soil isotope signatures, which is linked to the uptake of bioaccumulative MeHg that is produced under anoxic conditions prevalent in wetlands (Yin et al., 2013). The unique case of rice, particularly in ASGM affected areas, is considered separately in Sect. 3.2. We will now consider the impacts of ASGM-derived Hg contamination in crops via stomatal assimilation.
3.1.3 Hg uptake from air in crops impacted by ASGM activities
Eboigbe et al. (2025) assessed both air and soil uptake pathways in cassava (Manihot esculenta), peanut/groundnut (Arachis hypogaea), and maize (Zea mays) from a contaminated (≈500 m upwind) and a background (≈8 km upwind) farm of a ASGM processing site in Nasarawa State in Nigeria. Foliage was enriched 25–35× in the contaminated farm (compared to background), and Hg stable isotope analyses revealed highly negative MDF values in foliage (δ202Hg: cassava: −3.83 ‰ ± 0.15 ‰, peanut: −3.77 ‰ ± 0.27 ‰, maize: −2.51 ‰ ± 0.15 ‰), which are indicative of the negative fractionation associated with stomatal assimilation (Eboigbe et al., 2025). Air-to-foliage enrichment factors (ε202Hg3: −2.89 ‰ to −1.57 ‰) fell into the aforementioned measured range observed in other higher vegetation (Eboigbe et al., 2025). A two endmember Hg stable isotope mixing model based on air and soil uptake pathways revealed 61 %–100 % of THg in edible tubers/nuts/grains and other above ground tissues and 26 %–47 % of THg in roots were derived from air highlighting the dominance of the atmospheric uptake pathway in these crops. The fraction of MeHg out of THg was <1 % (%MeHg) in all measured crop and soil samples (Eboigbe et al., 2025). While THg and MeHg concentrations in edible parts were below dietary guidelines, without any data for Nigeria, conservative consumption rate estimates were used for cassava leaves (320 ± 116 µg kg−1); suggested consumption rates from other countries would have surpassed dietary intake thresholds (Eboigbe et al., 2025).
Casagrande et al. (2020) examined ASGM-derived Hg in soy plants (Glycine max) and found THg concentrations in leaves from plants grown in a ASGM affected area (mean THg: 109 ± 21 µg kg−1) approximately three times higher than soy foliage in more background sites (THg means: 35–40 µg kg−1). This was despite measuring relatively low soil THg concentrations in both ASGM (95 µg kg−1) and non-ASGM areas (68 µg kg−1); and indeed, THg concentrations in other plant tissues (stems, seeds, pods, and roots) were not elevated in the ASGM affected area (Casagrande et al., 2020). The authors link these results to atmospheric Hg uptake and used the data to estimate a Hg deposition/accumulation rate of this ASGM affected soy farm of 33.6 (Casagrande et al., 2020). This approach provides a novel basis for calculating Hg accumulation from air in both background and Hg contaminated agricultural areas. Eboigbe et al. (2025) also applied the Hg accumulation approach and calculated fluxes of 1070 ± 88, 98 ± 26, 620 ± 140 to cassava, peanuts (groundnuts), and maize farms, respectively. These estimates include transfer to other tissues including below ground edible parts, but Hg storage in foliage makes up the majority of Hg transferred to crops from air (90 %–92 %), which again raises concerns about consumption of edible foliage, such as in cassava (Eboigbe et al., 2025).
Several other studies have assessed Hg in crops from ASGM affected areas but did not make atmospheric Hg(0) measurements due either to logistical challenges or to the assumption that Hg would derive largely from soil. While less ideal than paired soil and atmosphere measurements, soil THg concentrations represent acceptable proxies for general Hg exposure across Hg(0) contaminated areas, as deposition from air is a major source of soil Hg, and Hg(0) in air typically correlates well with soil THg concentrations (Fantozzi et al., 2013; Xia et al., 2020). However, we acknowledge that there can be exceptions to this relationship including in ASGM areas (Gerson et al., 2022); and hence acknowledge the elevated uncertainty such an assumption creates.
Golow and Adzei (2002) measured THg concentrations up to ≈35 and ≈18 µg kg−1 in cassava leaves and flesh, respectively, at ≈2–3 km from a mining site in Ghana; concentrations in tissues and soils decreased with increasing distance from the ASGM site. However, these concentrations were low compared to most other studies (Table 1). Nyanza et al. (2014) observed THg concentrations of cassavas up to 167 µg kg−1 in leaves, but only up to 8.3 µg kg−1 in flesh (little specific information relating to distance from ASGM was given). Adjorlolo-Gasokpoh et al. (2012) measured elevated THg concentrations in both cassava leaves (up to 177 µg kg−1) and flesh (up to 185 µg kg−1) near another ASGM site in Ghana. While leaf THg concentrations were again reported to decrease with distance from mining sites, there may have been multiple sources in this study (i.e., former mines; Adjorlolo-Gasokpoh et al., 2012). A unique aspect of the Adjorlolo-Gasokpoh et al. (2012) study was that they dissected the cassava into flesh and inner and outer peels of the tuber and data from such tissue dissection could provide critical information in discerning atmospheric and soil uptake pathways. Nonetheless, there was little trend with distance from ASGM site in flesh, inner peel, or outer peel (Adjorlolo-Gasokpoh et al., 2012), which could be attributed to variability in the use/emission of Hg and possible unknown sources. Our own analyses of data from Nyanza et al. (2014; p=0.111) and Adjorlolo-Gasokpoh et al. (2012; p=0.136) indicate there was no correlation between THg concentration in cassava leaves and flesh in these studies, which is surprising considering that stable isotope data from Eboigbe et al. (2025) indicated the atmosphere as the source of Hg in cassava flesh.
Table 1Information from studies of crops farmed in non-saturated soils in agricultural areas impacted by ASGM activities. Tissue abbreviations: F – foliage; S – stem; R – root; T – tuber/fruit; N – nut. [ ] denotes concentration. NA: data not available.
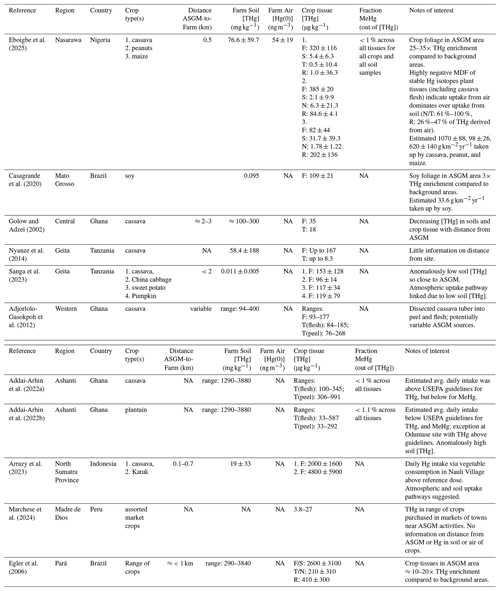
Addai-Arhin et al. (2022a) measured higher THg concentrations in both the peel (306–991 µg kg−1) and flesh (100–345 µg kg−1) of cassavas at farms near (specific distance not given) three ASGM sites in Ghana. MeHg concentrations were measured in cassava tissues and were <1 % of THg in all samples (Addai-Arhin et al., 2022a). In another study by the same group, Addai-Arhin et al. (2022b) measured both THg (and MeHg: <1.1 % of THg in all samples) in plantain (genus: Musa) flesh and peels at the same sites. THg concentrations in plantains (39–50 µg kg−1 in flesh and 41–130 µg kg−1 in peels) were close to an order of magnitude lower (Addai-Arhin et al., 2022b) than cassava (Addai-Arhin et al., 2022a) at the equivalent farms, which highlights the species specificity of Hg uptake in crops. In the 2021 study, much higher THg concentrations were observed in plantain flesh (mean: 580 µg kg−1) and peels (mean: 275 µg kg−1) at an additional fourth farm (Odumase) adjacent to what is [presumably] a much larger ASGM operation (Addai-Arhin et al., 2022b). Interestingly, the soils at Odumase site had lower THg concentrations than soils at other farms in their study (Addai-Arhin et al., 2022b); we speculate that the elevated THg concentration in plantain tissues at the Odumase farms is caused by greater emissions concentrations of Hg(0) in air from a potentially newer mine near this farm that may, as yet, not have impacted the soils as much as has been the case at other farms (no Hg(0) measurements were taken to assess this).
In both studies by Addai-Arhin et al. (2022a, b) human health assessments were included and based on USEPA daily consumption guidelines for THg in food (reference dose: 0.3 µg of Hg per kg of body mass per day; USEPA, 2004) and estimated average daily consumption rates (adults: 0.37 kg plantain, 0.6 kg cassava; children: 0.2 kg plantain, 0.4 kg cassava). The Hg consumption via cassava at all farms (measured range: 0.98–3.8 ; Addai-Arhin et al., 2022a) exceeded THg intake guidelines, but plantain only exceeded at the most contaminated farm (Odumase; 3.0–3.3 ; range at other farms: 0.22–0.28 ; Addai-Arhin et al., 2022b). While data are concerning, this may be partially offset by the low fraction of highly toxic and bioaccumulative MeHg, all cassava and plantain samples being below the USEPA daily MeHg consumption guideline (reference dose: 0.1 ; measured: <0.026 ; USEPA, 2004; Addai-Arhin et al., 2022a; 2022b). A third study by the Addai-Arhin et al. (2023) group appears to summarize these two other works, but it is not considered for further discussion here due to their focus on cumulative peel and flesh THg concentration data (sum of THg concentration in peels and flesh), which are not summative data.
Sanga et al. (2023) measured THg concentrations in edible crop foliage (cassava, pumpkin: Cucurbita moschata, Chinese cabbage: Brassica rapa subsp. pekinensis, and sweet potato: Ipomea batata) in crop soils indicative of anomalously low Hg contamination, near background levels (11.4 ± 4.7 µg kg−1), but <2 km from an ASGM area in Geita Region of Tanzania. THg concentrations were elevated and ranged from 96 ± 14 µg kg−1 in Chinese cabbage to 153 ± 128 µg kg−1 in cassava leaves.4
A similarly designed study in two villages in North Sumatra Province, Indonesia, Arrazy et al. (2023), also measured elevated THg in foliage of cassava (mean: 2000 ± 1600 µg kg−1) and katuk (Sauropus androgynus; mean: 4800 ± 5900 µg kg−1) foliage5; one village had dietary intakes from these leafy vegetables (0.52–0.93 ) above reference dose levels. However, the major difference to the Sanga et al. (2023) study was the ≈3 orders of magnitude higher THg concentrations in crop soils (mean: 19 ± 33 mg kg−1). The elevated THg concentrations in crops from both studies were hypothesized to be at least partly associated with atmospheric uptake, though no air measurements were taken (Sanga et al., 2023; Arrazy et al., 2023). Both studies also examined rice, discussed in Sect. 3.2.2.2.
A recent study in the Madre de Dios Region of Peru, examined the edible parts of six crops (corn: Z. mays, rice: O. sativa, cassava: M. esculenta, plantain: M. paradisiaca, potato: Solanum tuberosum, cocona: Solanum sessiliflorum) in areas deemed to be impacted by mining (Marchese et al., 2024). Concentration levels in crops from areas listed as “impacted by mining” were lower than in many of the previously mentioned studies ranging from 3.8 µg kg−1 (n=2) in corn to 27 µg kg−1 (n=2) (Marchese et al., 2024). Even so, four of the 27 samples exceeded maximum contaminant levels as indicated by the US Dept of Agriculture (Marchese et al., 2024). However, these crop samples were purchased in local markets presenting challenges in assessing distance from farms to mining sites and crop exposure levels to Hg from either soils or air (Marchese et al., 2024). Again, rice data from this study are interpreted in Sect. 3.2.2.2.
One other study from South America (Pará State, Brazil) attempted to correlate THg in both roots and above ground parts from a range of cultivated crops (grouped as produce) with soil THg (no assessment of Hg(0) in air) at two ASGM impacted communities (Egler et al., 2006). The first community appears to be a village setup around a mine (we assume farms are very close to mine) and THg concentrations were the highest measured across all studies examining Hg in crops impacted by ASGM (mean THg concentrations: 2600 ± 3100, 210 ± 310, and 410 ± 300 µg kg−1 in above ground parts, edible parts, and roots, respectively, across all crops). At the second site (≈15 km from active ASGM sites) THg in produce was lower (120 ± 110, 10 ± 10, and 260 ± 250 µg kg−1, respectively) and only produce roots at this location were significantly correlated with soil THg, which again suggests that atmospheric uptake is the dominant uptake mechanism for these crops (Egler et al., 2006).
Hg concentrations in crops have been assessed in several other studies. However, these papers lack details of sampling sites/methods and distance from ASGM (i.e., Essumang et al., 2007), contain unclear or concerning analytical methods (Essumang et al., 2007; Ahiamadjie et al., 2011), or had potential errors in data reporting (SSenku et al., 20236). Therefore, these studies are not considered further.
3.2 Hg uptake from roots of saturated soil crops: the drinkers
While stomatal assimilation of Hg(0) can and does occur in rice (Oryza sativa L.; Qin et al., 2020; Tang et al., 2021a; Aslam et al., 2022), rice is exceptional in that it also accumulates significant amounts of Hg from the soil, due to the availability of MeHg which is formed in the anaerobic paddy soils (Rothenberg et al., 2014). MeHg represents 40 %–60 % of the THg burden in rice (Rothenberg et al., 2014), which contrasts other crops that usually accumulate only 0.05 %–1 % MeHg even in contaminated areas (Qiu et al., 2008; Sun et al., 2020; Eboigbe et al., 2025). Rice is a staple food crop for >3.5 billion people (Zhao et al., 2020) and, globally, rice represents 10 % of total MeHg intake (Liu et al., 2019), emphasizing the considerable public health concerns posed by the consumption of MeHg and IHg(II) contaminated rice.
3.2.1 Rice paddies: the (de)methylators
Rice paddies are characterized by cyclical flooding and drying cycles. These cycles impact redox conditions, the forms of carbon (C), sulphur (S), iron (Fe), and manganese (Mn) cycling, and induce strong mineral weathering (Kögel-Knabner et al., 2010). In addition, rice paddies usually have abundant organic matter from root exudates and the reincorporation of rice residues. The soil pool of MeHg is the dominant source of MeHg to the plant, with multiple studies observing no evidence that in-planta methylation can occur (Aslam et al., 2022; Liu et al., 2021a; Strickman and Mitchell, 2017). MeHg in soil is governed by IHg(II) bioavailability and methylation and demethylation rates, while there are multiple pathways of MeHg and IHg(II) uptake into the roots and subsequent translocation into the grain, processes described in detail below.
Inorganic Hg (IHg(II)) bioavailability
The rapid redox cycling created by fluctuating water conditions in rice paddies can create “Hg species resetting” which increases the supply of soluble IHg(II) species (bio)available for methylation (Liu et al., 2021a; Wang et al., 2021a). Logically, this supply of (bio)available, soluble IHg(II) increases in paddies contaminated with Hg from anthropogenic activities (including ASGM) (Ao et al., 2020; Rothenberg et al., 2014; Xu et al., 2024). Other factors such as lower pH, oxidation of Fe(II) to Fe(III) via radial oxygen loss from rice roots, and application of N fertilizers, can also free IHg(II) from binding sites and increase its bioavailability for methylation (Rothenberg et al., 2014; Tang et al., 2020).
Methylation
Mercury methylators in rice paddies appear to be dominated by iron reducers (Liu et al., 2018; Tang et al., 2021b), methanogens (Liu et al., 2018; Tang et al., 2021b; Wu et al., 2020), and (in some cases) sulphur reducers (Wu et al., 2020). Several aspects of the rice paddy system influence methylation rates, with marked differences observed across geographical and contamination gradients (Liu et al., 2021a; Rothenberg et al., 2012). Methylation is stimulated by the availability of labile organic carbon, which originates from root exudates or rice straw debris (Liu et al., 2016; Windham-Myers et al., 2009; Zhu et al., 2015). In addition, the draining cycle of paddies facilitates oxic regeneration sulphate and ferric iron, electron acceptors of sulphur- and iron-reducing bacteria, as well as promoting dissolution of iron oxyhydroxides and thus release of bound IHg(II) (Rothenberg et al., 2014; Ullrich et al., 2001; Wang et al., 2021a).
Demethylation
Hg demethylation in rice paddy soil has been seldomly measured, but most studies report relatively high and consistent demethylation rate constants, suggesting resilience to different environmental conditions (Liu et al., 2021a; Windham-Myers et al., 2013; Zhao et al., 2016). The taxonomic diversity of Hg demethylators may explain this, as both mer-dependent and mer-independent demethylation have been observed in paddy soils, with evidence for demethylation by representatives of Clostridium spp. (Wang et al., 2021a), Catenulisporaceae, Frankiaceae, Mycobacteriaceae, and Thermomonosporaceae (Liu et al., 2018). Correlations between MeHg concentrations and methane emissions from paddies suggest methanogens are important demethylators (Huang et al., 2025). Demethylation appears to be responsive to labile organic carbon (Marvin-DiPasquale and Oremland, 1998; Marvin-DiPasquale et al., 2000; Hamelin et al., 2015; Li and Cai, 2012), but less so than methylation, based on a comparison of methylation and demethylation in vegetated and devegetated plots of rice paddies, which observed concomitant increases in plant-derived labile organic carbon, MeHg concentrations, and methylation rate. Demethylation was not measured, but any increases in this process had to have been outpaced by the increase in methylation rate (Windham-Myers et al., 2013).
Uptake and translocation of MeHg, IHg(II), and Hg(0) through the plant-grain system
The uptake routes of MeHg and IHg(II) to rice differ substantially. MeHg is formed in the soil and then absorbed through the roots; a fraction of this MeHg is retained by iron plaque or apoplastic barriers on the root tissue, preventing complete transfer of MeHg to internal root vascular tissues and subsequent translocation (these barriers can also prevent IHg (II) uptake into internal tissues) (Li et al., 2015; Wang et al., 2014, 2015; Zhou and Li, 2019). The review by Rothenberg et al. (2014) confirmed greater uptake of MeHg in rice by calculating average bioaccumulation factors from previously published works of 5.5 for MeHg and 0.32 for IHg(II). While there is uncertainty around the exact mechanisms driving translocation, it likely occurs through conductive tissues such as phloem and xylem (Rothenberg et al., 2015; Hao et al., 2022; Meng et al., 2010, 2014; Xu et al., 2016).
Within above ground tissues, MeHg can be photolytically demethylated via reactive oxygen species generated by the plant itself (Li et al., 2015; Strickman and Mitchell, 2017; Xu et al., 2016). In-planta demethylation can eliminate up to 84 % of the MeHg absorbed from the soil by rice (Tang et al., 2025) which is responsible for a protective effect valued at USD 30.7–84.2 billion per year (Tang et al., 2024). Translocation of MeHg to the rice grain appears to occur in complex with cysteine residues and concentrated in the endosperm (the “white” core of the rice grain) (Meng et al., 2014). Rice grains are referred to throughout as either unhulled, once-milled (husk removed, bran not removed; brown rice) or twice-milled (husk and bran both removed; white rice).
IHg(II) can also be taken up by plants in similar pathways described in Sect. 3.1. Sorption of IHg(II) to roots has been observed in rice (Aslam et al., 2022; Liu et al., 2021a; Strickman and Mitchell, 2017), but similar to other crops the root epidermis likely restricts assimilation of IHg(II) into internal root tissues limiting translocation to other tissues via this uptake pathway. Similar to MeHg, iron plaque coatings on rice roots contribute to the root barrier for IHg(II) via adsorption (Li et al., 2015; Wang et al., 2014, 2015; Zhou and Li, 2019). Stomatal assimilation of Hg(0), subsequent oxidation, and translocation has been observed as a source of IHg(II) to the developing rice grain (Aslam et al., 2022; Liu et al., 2021a; Yin et al., 2013) as well as to the roots themselves via reverse translocation (Aslam et al., 2022). It has also been posited that some IHg(II) could sorb to the outer layers of the grain (bran and aleurone layer) directly from the atmosphere (Meng et al., 2014).
3.2.2 Hg in rice impacted by ASGM activities
Globally, Hg contamination of rice in contaminated and uncontaminated areas has been reviewed by Rothenberg et al. (2014) and Tang et al. (2020), and in Indonesia by Arrazy et al. (2024). Our review integrates the ASGM-related body of this research with newer findings to update our understanding of ASGM impacts on rice. We note the importance of understanding ASGM-derived Hg contamination of rice due to the prevalence of ASGM in rice growing areas (i.e., Asia and Africa), the resulting Hg contamination of air, soils, and water, and the presence of Hg(0), IHg(II), and MeHg in these paddy systems.
Assessment of methylmercury production in ASGM impacted paddy systems.
Rates of methylation and demethylation have never been estimated in ASGM environments, and only one study has measured MeHg levels in paddy soil/sediments. Working in West Java, Indonesia, Tomiyasu et al. (2020) measured mean MeHg concentrations of 12.3 ± 4.8 µg kg−1 in paddy soils ≈500 m downstream from an ASGM site compared to 6.5 ± 2.12 µg kg−1 in reference paddy soils ≈12 km upstream, which seems to indicate minimal differences in methylation between ASGM and non-ASGM environments. However, accounting for the THg concentrations in soils (0.43 ± 0.07 mg kg−1 and 17.4 ± 22.5 mg kg−1 at the reference and ASGM-impacted paddies, respectively), %MeHg levels were highest at the reference site (1.6 ± >0.1 %) compared to 0.1 ± 0.15 % at the ASGM impacted paddy (Tomiyasu et al., 2020). These observations suggest that differences in the biogeochemical drivers of methylation/demethylation could be more important to MeHg concentrations than THg concentration in these systems, and that methylation was low and/or demethylation was high at the ASGM paddy site. Predominant winds and potential atmospheric uptake of Hg(0) could also be a factor if upstream paddies were downwind, because the speciation of Hg could alter bioavailability for methylation, but these details were not provided.
What do we know about methylmercury accumulation in rice in ASGM areas?
As for other foodstuffs, the tolerable daily intake rate (the reference dose) of THg and MeHg in rice are related to the composition of the entire diet, other MeHg sources, the duration of exposure and the weight of the individual. While there are concerns that rice should have a separate reference dose, because it does not offer the same beneficial micronutrients as fish (Rothenberg et al., 2014), this work has not been undertaken. For consistency, we therefore use the same reference doses for THg and MeHg described in Sect. 3.1.3 (0.3 and 0.1 for THg and MeHg, USEPA, 2004) for studies that discuss estimated dietary intakes and that presented their intake calculation method. Some authors incorporated a wet to dry correction factor to their intake calculations, which we report, if present, since different correction factors can affect final values. For studies that did not assess dietary intake, did not report their calculation method, or did not distinguish rice from other sources of MeHg, we contextualize the health risk using the Chinese maximum allowable concentration (MAC) for THg in rice, set at 20 µg kg−1 (Zhao et al., 2019). As there are no MAC values for MeHg in rice, we apply the same MAC of 20 µg kg−1 for MeHg; if the more toxic and bioaccumulative MeHg concentrations exceed this threshold they assuredly present human and environmental health concerns. For context, the global averages for THg and MeHg levels in rice from uncontaminated areas are 8.2 and 2.5 µg kg−1 respectively (Rothenberg et al., 2014).
Information on MeHg in rice grain in ASGM areas is limited. Findings vary widely, from minimally contaminated (1–2 µg kg−1) to levels of high concern (over 100 µg kg−1). These values are within the same order of magnitude as previous findings of MeHg in rice grains from contaminated paddies associated with other anthropogenic Hg sources (1.2–63 µg kg−1, Rothenberg et al., 2014).
Two authors employed a market-basket approach, where rice grains were purchased in regions around ASGM activities. In addition to data on other crops (see Sect. 3.1.3), Marchese et al. (2024) observed similar MeHg and THg levels in rice grain in mining-impacted (MeHg: 7.9 ± 7.17 µg kg−1, THg: 9.1 ± 2.9 µg kg−1) compared to non-mining-impacted areas (MeHg: 8.7 ± 7.5 µg kg−1, THg: 15.2 ± 19.9 µg kg−1). However, it was not possible to link these market basket samples to contamination in individual mining-adjacent paddies, as the specific growing location was unknown (Marchese et al., 2024). The same concerns about unknown paddy locations persisted in a study by Cheng et al. (2013) in Cambodia, who observed mean MeHg concentrations of 1.54 kg−1 in market rice bought in a mining-intensive district compared to means of 1.44 and 2.34 µg kg−1 in non-mining districts. %MeHg was not calculated for individual samples, but using overall mean THg and MeHg values, we estimate that the %MeHg in the ASGM area was low, at ≈12 %, and similar to the %MeHg values from non-mining regions (≈20 %; Cheng et al., 2013). These studies suggest that the local commercial rice supply is relatively homogenous between mining- and non-mining areas, which limits the effectiveness of market basket studies for determining Hg exposure of vulnerable populations (miners and local residents) via rice in ASGM regions.
Two authors explored MeHg in rice grains derived from farms/paddies situated in close proximity to ASGM sites. Novirsa et al. (2020) found THg concentrations (mean: 48.5 µg kg−1; range 13.8–115 µg kg−1) in locally grown rice in active ASGM and farming community (amalgamation “Hg hotspot” ≈500 m from rice paddy) in Lebaksitu, Indonesia that exceeded the Indonesian standard of 30 µg kg−1 for Hg in foodstuffs; of this, 15 %–82 % (mean: 41 %) was MeHg (mean: 14 µg kg−1). Rice THg concentrations in a second village approximately 2000 m from “Hg hotspot” were lower (mean: 15.9 µg kg−1; range 9.1–23.2 µg kg−1), as was the MeHg concentration (mean 9.8; range 6.5–11.7 µg kg−1) but %MeHg increased (mean: 65 %; range: 51 %–80 %) (Novirsa et al., 2020). The authors intuitively link the difference in %MeHg to greater proportional uptake of atmospherically deposited inorganic Hg (we suggest predominantly via stomatal assimilation of Hg(0)) by rice plants grown closer to the “Hg hotspot” (Novirsa et al., 2020). These authors estimated the probable daily intake (which incorporates an estimate of bioavailability) of MeHg from rice and found that intake exceeded the reference dose in the nearer village (0.139 µg kg(bw)−1 d−1, range 0.079–0.199) while intake in the father village fell below the threshold (0.063 µg kg(bw)−1 d−1, range 0.040–0.093). In addition, they found a significant correlation between hair MeHg levels and exposure via rice, indicating that the contaminated rice was the source of the residents' MeHg intake (Novirsa et al., 2020).
In their companion paper in the same area, Novirsa et al. (2019) reported very high THg concentrations in soils at the “Hg hotspot” (32.1 mg kg−1; n=1). A negative correlation between THg concentrations and distance from source (three sites between 0.25 and 1.5 km from the hotspot) was also observed in paddy soils (from 2.26 to 0.47 µg kg−1), paddy waters (from 301 to 30 ng L−1), and rice grains (from 212 to 29 µg kg−1) (full details in Table 2) (Novirsa et al., 2019). Yet they found no relationship between soil or grain THg and water THg levels (Novirsa et al., 2019). Interestingly, this paper identified a positive correlation between soil THg and grain THg, but the authors did not statistically relate these THg measurements to MeHg measurements in their later work, limiting conclusions that can be made about the relationship between THg and MeHg contamination (Novirsa et al., 2019).
Table 2Summary of studies examining Hg in rice. All data presented in means ± standard deviation if these data were available or could be calculated from tabulated datasets. If means ± standard deviations were not provided or could not be calculated, we provide the values supplied by the authors (means and ranges, mean only, or ranges only). For studies without measurements of rice grain MeHg, we have provided a coarse estimate of the MeHg content based on the rice grain THg values and the average %-MeHg value observed by Novirsa et al. (2020) (53 %); these values are marked with an asterisk. NA: data not available.
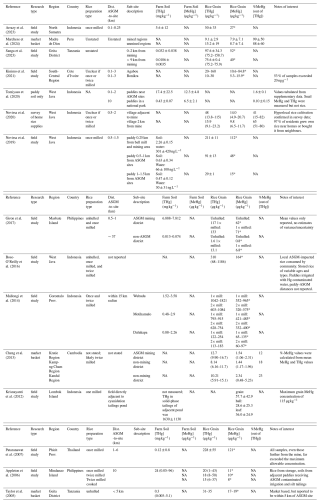
Elevated MeHg concentrations were measured in rice grains (mean: 57.7 ± 42.9 µg kg−1), husk (mean: 28.6 ± 25.3 µg kg−1), and foliage (mean: 36.0 ± 24.9 µg kg−1) from paddy fields directly adjacent to a very highly Hg contaminated ASGM cyanidation tailings pond (mean THg in dried solid-phase tailings: 1.63 ± 1.13 g kg−1) in Sekotong area on Lombok Island (Krisnayanti et al., 2012). THg was not measured in rice grains, and MeHg was not measured in the tailings ponds, making it difficult to compare estimates of methylation in soil to MeHg accumulation in grain (Krisnayanti et al., 2012). Nonetheless, the measured mean MeHg concentration in rice grains far exceeded the Chinese MAC of 20 µg kg−1 (Krisnayanti et al., 2012). The very high MeHg concentrations observed in these two studies highlight the elevated health risk associated with consumption of rice grown in areas impacted by ASGM activities.
What do we know about total mercury accumulation in rice in ASGM areas?
Given that MeHg is routinely detected in rice samples when sufficiently sensitive measurement techniques are used (Rothenberg et al., 2014), it is likely that MeHg contamination of rice grains in ASGM areas is widespread. To help aid with comparison between studies, we have included estimates of MeHg concentrations for all studies that have only assessed THg in rice (those discussed in this section) by multiplying the THg concentrations by the mean %MeHg in rice across both villages (53 % ± 12 %) from Novirsa et al. (2020) in Table 2. We emphasize that these estimates have a high uncertainty.
Concentrations of THg in rice grain have been assessed in ASGM areas of South America, Southeast Asia, and Africa, presented in Table 2. From the studies reviewed here, THg concentrations in rice in ASGM areas range from 1.0–1810 µg kg−1. This range exceeds that previously found by Rothenberg et al. (2014), who surveyed Hg in rice in control (mean 8.2 µg kg−1, range 1.0–45 µg kg−1) and contaminated areas (mean 65 µg kg−1; range 2.3–510 µg kg−1) impacted by Hg use in e-waste, cement production, and other industrial and mining activities, including some earlier studies on Hg in rice in ASGM areas. The literature summarized below excludes studies covered in the Methylmercury section (3.2.2.2.1), which includes the only work from South America (Marchese et al., 2024). In addition, several studies were excluded due to issues with quality control reporting or inconsistencies in data tabulation in text (Hindersah et al., 2018; Ramlan et al., 2022; Saragih et al., 2021; Ssenku et al., 2023).
Southeast Asia, particularly Indonesia, has received more attention than other regions, but levels of THg contamination were variable and did not always translate to elevated THg in rice. For instance, surprisingly low rice THg contamination was observed by Appleton et al. (2006), who studied Hg in waters, sediments, different types of agricultural soils, mussels, fish, bananas, and rice prepared in various ways in an irrigated farming area in the Naboc watershed, downstream of an ASGM site on Mindanao Island, the Philippines. Expectedly, irrigation of rice paddies with Hg-contaminated water from the mine resulted in significantly higher THg concentrations in paddy soils (mean: 24, range 0.05–96 mg kg−1) compared to unirrigated banana and corn field soils (means of 0.12 and 0.27 mg kg−1 respectively) (Appleton et al., 2006). However, rice Hg levels ranged from an average of 20 µg kg−1 for once-milled rice (range 1–43 µg kg−1), 18 µg kg−1 for twice-milled rice (range 8–50 µg kg−1) and 15 µg kg−1 for cooked twice-milled rice (range 6–37 µg kg−1) (Appleton et al., 2006). These results highlight that the preparation method of rice, including cooking, has the potential to modulate exposure risk. The authors suggested that the surprisingly low THg concentrations in rice, given the degree of soil contamination, could be the result of the post-harvest sampling strategy, which combined rice grown in paddies with variable magnitudes of contamination (Appleton et al., 2006).
In contrast, Pataranawat et al. (2007) conducted THg measurements of paddy waters, soils and rice (as well as other matrices) around an ASGM facility in Phichit Province, Thailand, and observed that once-milled rice had very high THg concentrations (228 ± 55 µg kg−1). However, the surface soil THg concentrations (unclear if this was paddy soil but associated with the rice samples: 120 ± 80 µg kg−1) were lower compared to other ASGM sites (Table 2) (Pataranawat et al., 2007). The authors also measured elevated Hg dry deposition rates in the area (range: 24–139 µg m2 d−1; compared to background dry deposition rates in Japan: 8.0 ± 2.7 µg m2 d−1; Sakata and Marumoto, 2005) and suggested stomatal assimilation of Hg as the explanation for the elevated rice and low paddy soil THg concentrations. However, the study lacked both MeHg measurements in rice or paddy soils (a significant fraction of the THg content of rice), and foliage Hg measurements to more comprehensively assess this hypothesis (Pataranawat et al., 2007).
Working in three villages within 15 km (specific distance of each village to ASGM site not listed) of an active ASGM site in North Gorontalo Province, Indonesia, Mallongi et al. (2014) observed very high THg concentrations in both once-milled (up to 1812 µg kg−1) and twice-milled rice (up to 1080 µg kg−1) (Table 2). Stomatal assimilation was again speculated as a potential contributor to the high THg concentrations in rice due to high measured dry deposition rates (166–219 µg m2 d−1) but the authors again lacked the appropriate analyses to confirm this (Mallongi et al., 2014). They also included a diet-based health assessment that raised concerns of residents consuming this rice in this area, particularly brown rice from the village closest the ASGM site (Mallongi et al., 2014).
Giron et al. (2017) surveyed the soil and rice grain THg concentrations on Masbete Island, the Philippines, at rice fields near an ASGM site, and a reference site ≈37 km away. They found that paddy soil THg concentrations were extremely elevated in the ASGM site (6880–7810 µg kg−1) compared to the distant region (13–74 µg kg−1). Unhulled and once-milled rice concentrations were also elevated at the ASGM site in comparison to the control site (117–133 and 1.6–13 µg kg−1, respectively; Giron et al., 2017). The ASGM site was directly adjacent to a tailings pond and reportedly received tailings contaminated water (Giron et al., 2017).
Arrazy et al. (2023) measured somewhat lower THg concentrations in rice (mean: 50 ± 33 µg kg−1) from similarly contaminated ASGM-derived Hg paddy soils (mean THg: 5600 ± 12 000 µg kg−1) in rice-growing villages with active amalgamation and amalgam burning North Sumatra Province, Indonesia. In this study, THg concentrations in rice were correlated with THg in soils and distance from amalgam burning sources, but all rice sources were 300–600 m from these sites; hence all sites were heavily contaminated (Arrazy et al., 2023). The authors also calculated average daily intake values of THg from rice for adults (0.30–0.34 ) and children (0.54–0.63 ) using a wet/dry conversion factor set at 0.91; both adults and children had exposures above the USEPA reference dose level (Arrazy et al., 2023).
A small epidemiological study exploring the health effects of mercury exposure in an ASGM village in Indonesia observed that the local rice supply, upon which the villagers depended entirely, was highly contaminated (68–1186 µg kg−1 of THg in unhusked, once-milled, and twice milled stored rice of various ages; mean value 301 µg kg−1), and estimated THg intake rates of 0.14 for adults and 0.57 for children (Bose-O'Reilly et al., 2016). Of the 18 villagers examined, 15 were experiencing symptoms of clinical Hg intoxication (Bose-O'Reilly et al., 2016). These affected individuals had relatively high THg levels in hair combined with relatively low THg levels in urine, which is indicative of the manifestations of MeHg exposure rather than inorganic Hg exposure (Bose-O'Reilly et al., 2016).
Shifting to Africa, studies of ASGM impacted rice paddy systems were typically indicative of lower concentrations of THg in paddy soils compared to studies in SE Asia. This may reflect more distributed cultivation of rice in Africa, greater competition for the same land resources in SE Asia, or simply that researchers have not been able to study more heavily impacted rice paddies in Africa due to social/geopolitical drivers or funding/capacity issues. Taylor et al. (2005) explored Hg in rice around a mining area in Nigeria using a market basket approach combined with a single paired rice-soil sample as part of a more complex survey of dietary metal contamination across multiple environmental compartments. They found that rice grown within 5 km of the ASGM site had THg concentrations of 31–35 µg kg−1 and Hg in these paddy soils had a mean THg concentration of 120 µg kg−1 (Taylor et al., 2005). However, other paddies that were not sampled for rice had much higher THg concentrations in paddy soils (up to 5100 µg kg−1) (Taylor et al., 2005); hence, the measured THg concentrations of rice may be on the low end of actual rice concentrations in this ASGM affected area.
Kinimo et al. (2021) assessed Hg contamination of rice and human exposure at two ASGM sites in rice-subsistence communities of Ababou and Bonikro, in south-central Cote d'Ivoire. In once-milled rice, THg concentrations were 20 ± 10 µg kg−1 at Bonikro (53 % of samples exceeded Chinese MAC threshold), and 40 ± 20 µg kg−1 in Agabou (all samples exceeded) (Kinimo et al., 2021). Nonetheless, calculated average daily intakes of Hg via rice fell below the USEPA threshold (Bonikro: 0.0075 , range 0.0029–0.016; Agabou mean 0.018 , range 0.0073–0.079). However, their wet/dry conversion factor was set to 0.085, an order of magnitude lower than that used by other authors here (Arrazy et al., 2023: 0.91, Sanga et al., 2023: 0.86) and may have biased these estimates (Kinimo et al., 2021).
Finally, Sanga et al. (2023), measured elevated rice grain THg concentrations (mean: 97.6 ± 34.3 µg kg−1) near (<2 km) an ASGM site in Geita Region of Tanzania and calculated a daily intake of Hg from rice of 0.429 using a wet/dry conversion factor of 0.86; both rice concentrations and intake rates exceed safe thresholds. Sanga et al. (2023) observed that rice grain THg concentrations (mean: 75.6 ± 0.005 µg kg−1) at a “background” site (≈9 km away) were also above the Chinese MAC (EDIs not estimated at this site). Despite the elevated Hg concentration in rice grains, paddy soil THg concentrations at both the near mining (mean: 32.1 ± 38.2 µg kg−1) and “background” (mean: 10.6 ± 2.3 µg kg−1) were anomalously low and near background levels (Sanga et al., 2023). Atmospheric foliar uptake was briefly discussed with relation to other crops examined in this study but not linked directly to the observed high rice Hg and low soil Hg data (Sanga et al., 2023). We posit that foliar uptake and translocation of IHg(II) to rice grains could drive this discrepancy.7
The literature summarized in this section suggest that both uptake through roots (likely of MeHg) and Hg(0) uptake through foliage are important determinants of grain THg concentrations in rice grains in ASGM areas. This conclusion is largely derived from the data inconsistencies between THg concentrations in paddy soils (and on occasion also distance from source) and THg concentrations in rice, which indicate that simple soil THg concentration was not the only control on grain THg concentration in grains (i.e., Appleton et al., 2006; Pataranawat et al., 2007; Sanga et al., 2023), as well as the comprehensively structured study by Aslam et al. (2022) which strongly suggested an atmospheric route of Hg(0) uptake is occurring in rice. This does not discount the importance of uptake from roots in ASGM areas, as there are studies that have observed a positive rice grain – paddy soil THg correlation (i.e., Arrazy et al., 2023; Novirsa et al., 2019). While the authors interpreted this to mean that the soil was the source of grain THg, we believe it is more likely to be the result of bioaccumulation of the (unmeasured) methylated fraction of the total Hg pool, given that MeHg is readily detected in rice grains at high levels in ASGM areas (Krisnayanti et al., 2012; Novirsa et al., 2020; Rothenberg et al., 2014). While we cannot fully discount the possibility of direct soil uptake of IHg, the presence of IHg in rice grain could also be explained by the recently confirmed in-planta demethylation pathway (Tang et al., 2024), or stomatal uptake and subsequent reverse translocation (Aslam et al., 2022) followed by loading to the developing grain. Studies to better understand the local controls over both uptake mechanisms, and why anomalously low rice Hg occurs in areas with high paddy soil Hg (and vice versa), should be the focus of future research
3.3 Hg uptake by livestock/poultry: the consumers
Restricting our definition of agriculture to more traditional terrestrial farming practices (fungi or aquaculture farming are not considered), we must also consider potential Hg exposures through the consumption of Hg contaminated livestock, poultry, or their egg/dairy by-products; yet research in this area is very limited. Hg in herbivorous, mammalian livestock (i.e., cattle, sheep) and their milk is suggested to be derived largely from Hg in feedstocks with inhalation deemed a minor uptake pathway (Vreman et al., 1986; Crout et al., 2004; Parsaei et al., 2019). Qian et al. (2021) mention that Hg speciation, and specifically the fraction of MeHg in the contaminated feedstocks is likely to impact the extent of bioaccumulation in poultry and livestock. Yet the authors did not directly measure any form of Hg in the animals or animal products (only THg and MeHg in plants) and simply highlight this potential exposure pathway (Qian et al., 2021).
Vreman et al. (1986) demonstrated that dosing cattle (Bos taurus) for 3 months with feedstocks enriched in inorganic Hg (1.2–3.1 mg of Hg per day) above control doses (0.2 mg of Hg per day) can result in accumulation of Hg in the animals, particularly in the liver (9× Hg enrichment in liver tissue vs control) and kidneys (16× Hg enrichment in kidney tissue vs. control). Similar results (Hg enrichment in kidneys and liver compared to muscle) were found by Crout et al. (2004) by dosing cattle feedstocks with isotopically labelled inorganic Hg, but no control cattle were used in this study. These data present livestock health implications due to the known impacts of Hg on the gastrointestinal and renal systems in humans and other mammals (Ha et al., 2017; Basu et al., 2023). Indeed, data demonstrating the concentration of Hg in the kidneys and liver of terrestrially farmed animals not only stress the need for caution/avoidance of human consumption of these tissues in regions with known Hg pollution issues such as ASGM areas, but they also highlight renal and gastrointestinal health risks in humans consuming of crops contaminated by inorganic Hg (via the stomatal assimilation pathway).
Hg in terrestrially farmed animals impacted by ASGM activities
Basri et al. (2017) measured significantly higher THg concentrations in hair of cattle living inside (<2 km from; 11.4 ± 9.5 mg kg−1) compared to outside (>8 km from; 2.9 ± 2.5 mg kg−1) an ASGM area on the island of Sulawesi. THg concentrations in hair also increased with cattle age, which suggests Hg is bioaccumulating the cattle (Basri et al., 2017). In a follow-up study of the same area, the authors examined soils and forage grasses (Imperata cylindrica, Megathyrsus maximus, and Manihot utilissima) that these cattle feed upon; though THg concentrations in soils were significantly higher inside compared to outside the mining area, the difference for forage grasses (inside vs outside) was not determined to be significant (Basri et al., 2020).
A study from Ghana examined liver, kidney, and muscle in sheep (Ovis aries), goat (Capra hircus), and chicken (Gallus gallus domesticus) and in each case THg concentrations were greater in kidneys (7 ± 8, 3 ± 2, and 12 ± 8 µg kg−1, respectively) than liver (3 ± 3, 1 ± 1, and 11 ± 7 µg kg−1, respectively), which were higher again than muscle (non-detect, non-detect, and 1 ± 1 µg kg−1, respectively) (Bortey-Sam et al., 2015). While the study did use a robust and highly sensitive THg analyser (MA3000, NIC), it appears low sample mass impacted the detectable THg concentration in the results (Bortey-Sam et al., 2015). Furthermore, chickens were market bought, and sheep and goat were obtained from slaughterhouses; hence, little specific information on feed and exposures could be determined (Bortey-Sam et al., 2015).
Marchese et al. (2024) assessed THg in feathers, eggs, and internal tissues (muscles and organs) and MeHg in eggs and internal tissues of “backyard” chickens from an ASGM community and an upstream remote community in the Peruvian Amazon (Madre de Dios Region). Median THg concentrations were 7.3× higher in muscle and organ tissues and 3.6× higher in feathers from mining areas compared to the background site; there was no significant difference in egg THg or MeHg between the sites (Marchese et al., 2024). Interestingly, chicken livers had the highest THg concentration, but lowest fraction of MeHg (54 %; MeHg fraction was up to 100 % in other tissues: spleen and back muscle) and MeHg fractions were significantly lower in ASGM area than background (Marchese et al., 2024).
The omnivorous nature of chickens and other poultry presents additional dietary variables to their own and subsequent human (via consumption of meat and eggs) exposures to Hg; their diets can vary greatly depending on how they are reared (Klasing, 2005). Indeed, Marchese et al. (2024) observed significantly higher δ13C data in chicken feathers in background area compared to ASGM area, suggesting differences in chicken diets between the sites. The lack of difference in δ15N between the sites indicates that this is not associated with a significant change in trophic feeding level but rather changes in plant food types (Marchese et al., 2024). Despite these differences the authors conclude that differences in environmental exposure levels drive the observed differences in chicken THg and MeHg concentrations at the ASGM and background sites (Marchese et al., 2024). In addition to Hg in chicken and crops, the Marchese et al. (2024) study also examined Hg in fish and combined all these data to produce probable weekly Hg intake values for humans in these regions. As expected, fish are the dominant dietary source of Hg make up ≈82 % of THg intake (≈96 % of MeHg) compared to ≈17 % (≈3 %) and ≈1 % (≈1 %) for crops and chicken, respectively (Marchese et al., 2024). Although the high THg concentration and lower MeHg fractions observed in chicken tissues (particularly livers) again raises some concern of inorganic Hg contamination and potential bioaccumulation in (particularly in detoxifying organs of) poultry/livestock in ASGM affected areas, the much larger Hg burden from fish consumption adds crucial perspective to dietary concerns relating to poultry/livestock consumption at least based on results of the Marchese et al. (2024) study.
Two other studies have examined THg concentrations in poultry blood. Abdulmalik et al. (2022) measured significantly higher THg blood concentrations (0.08–0.09 µg L−1) in chickens sampled within 1 km of ASGM compared to control chickens (non-detectable concentrations). While Aendo et al. (2022) measured much higher THg concentrations in poultry blood (mean THg range: 20–43 µg L−1), linkages between concentrations and proximity to mining were less clear. Only free-grazing ducks (specific species not listed) within a mining area (albeit a large area, within 25 km radius, deemed to be impacted by mining) had significantly higher THg concentrations to those outside the mining area; chickens and farmed ducks were not significantly different (Aendo et al., 2022).
The global extent and rapid growth of ASGM places critical emphasis on the need to address the serious environmental and human health risks presented by ASGM Hg use. Ideally, such efforts should start with improving our understanding of Hg emissions and releases associated with ASGM, which are highly uncertain and currently based on poorly constrained knowledge of Hg use, gold production, and the sheer scale of the rapidly growing and largely informal/illegal sector. The implementation of accessible, low-cost, low-tech solutions such as the Hg passive air sampler method utilized by Szponar et al. (2025) to assess Hg(0) concentrations, exposures, and emissions to air from ASGM activities are needed to generate the robust monitoring data needed to better assess ASGM Hg emissions and releases. Efforts to model ASGM emissions and fate remain hampered by our limited knowledge of Hg use inventories. Nonetheless, novel ASGM Hg modelling efforts that account for the importance of the sink of Hg to terrestrial vegetation (particularly in the more heavily vegetated tropics where much ASGM occurs) such as that presented by Hedgecock et al. (2024) will undoubtedly improve our understanding of the cross-compartmental distribution and air-vegetation dynamics of Hg in ASGM areas. Considering >55 % of the planet's ice-free land has been converted to farming or lands for human settlement (Ellis et al., 2010), it could be beneficial to adapt such models to include agricultural biomes.
There have been considerable advancements, paradigm shifts even, in terms of our understanding of the importance of Hg(0) uptake (stomatal assimilation) by plants from the atmosphere, now understood to be the dominant flux of Hg from air to terrestrial systems. However, there needs to be a greater focus on such research from the context of ASGM and agricultural crops. The recent work by Eboigbe et al. (2025) using Hg stable isotopes analyses of soils, air, and different crop tissues provided critical insight into the importance of the stomatal assimilation pathway in staple crops. While many previous studies of Hg in crops mention this as a potential uptake mechanism, this research has largely focussed on soil contamination as the primary source of crop exposure to Hg. Experimental design of future research should not discount soil uptake entirely, definitely not in the context of MeHg uptake in rice but assessment of the atmospheric Hg(0) concentration crops are exposed to should be an essential component of future studies in this area. Again, more accessible air monitoring technologies such as passive sampling are likely the most effective strategy considering that most ASGM happens in the Global South. Such data are not only critical for assessment crop exposures to atmospheric Hg, but also to assess the magnitude of ASGM emissions at specific sites (Szponar et al., 2025). As posited by Arrazy et al. (2024) and Rothenberg et al. (2014) the types of ASGM activities and the intensity and age of those activities as influencing factors on crop Hg concentrations and speciation.
The complexity of MeHg production, and paddy cycling of Hg, have been under appreciated in ASGM environments. Including such analyses in future work would improve interpretation of studies that observe anomalous data of low soil and high rice THg concentrations (and vice versa). Future work should incorporate measurements of Hg(0) at the studied paddies to assess atmospheric exposures of rice to Hg(0) and delineate the burden of THg in rice coming from air-stomata uptake pathway (and potentially direct sorption of atmospheric Hg species to developing grain). Adding measurements of MeHg in soil and grain compartments would allow greater capacity to differentiate if anomalous high soil/low rice or low soil/high rice THg concentrations are driven more by variable methylation rates in different paddies or a greater fraction of THg in rice being derived from the Hg(0) stomatal assimilation pathway than previously thought.
Authors focussed on concentration data and seldom measured the biogeochemical factors that could help explain and understand methylation in ASGM rice paddies. Data on relevant soil and water biogeochemistry is limited to nearby waterways, rather than paddies (Appleton et al., 2006; Pataranawat et al., 2007). Where feasible, measurements of methylation and demethylation rate potentials, Hg stable isotopes (or isotope enrichments), and complementary biogeochemical analyses (i.e., pH, temperature, redox conditions, carbon composition) are also needed. It is important to note that even if methylation rates are low, the extremely high supplies of inorganic mercury in ASGM environments can still lead to high concentrations of MeHg; this question remains largely unexplored. These knowledge gaps of Hg cycling in ASGM impacted paddy soils limit our capacity to identify specific drivers of elevated MeHg production and the associated health risks. This in turn makes it difficult to identify which agricultural strategies that have potential to reduce paddy production of MeHg and accumulation in rice grains (i.e., biochar amendment, alternative wetting and drying cultivation, or the use of low-MeHg accumulating cultivars; Tang et al., 2020).
We must consider that the range of crops potentially affected by ASGM activities is broad. C3 and C4 plants have different photosynthetic pathways, which as Eboigbe et al. (2025) speculate could lead to differing rates of Hg(0) uptake from air. Xia et al. (2020) suggest longevity of crops (annuals vs. perennials) may also impact Hg uptake rates from air and/or soils. Future work should not only broaden the range of crop species exposed to Hg contamination from ASGM, but also as many different crop tissues, beyond simply edible parts, as possible, and even different compartments of individual tissues (i.e., tubers: peels vs. flesh; stems and roots: cortex/epidermis vs. vascular bundles vs. pith). Such detail is crucial for subsurface tissues (i.e., roots) as it has been suggested that the root epidermis is an effective barrier preventing uptake of inorganic Hg species (Lomonte et al., 2014). Applying Hg stable isotope analyses to the different sections of dissected tissues has the potential to identify the source of Hg in each tissue section using two end-member mixing models for the air and soil uptake pathways (as applied in Eboigbe et al., 2025) as well as elucidate information on the internal translocation of Hg by these crops. Development of a process-based vegetation model examining internal Hg cycling using THg, Hg(0), IHg(II), and MeHg concentrations and stable isotopes (including fractionation factors) would be a major advancement not just for ASGM impacted farming systems, but for all study of Hg in vegetation.
It is a clear from our review that there is a dearth of information relating to Hg in livestock and poultry meat and dairy/egg by-products be that in high-risk ASGM areas or otherwise. Concerns of inorganic Hg bioaccumulation and health impacts are evidenced by Hg in livestock and poultry, particularly in detoxifying organs like kidneys and the liver. More study is required to understand the health risks to livestock/poultry themselves and humans consuming them (and their edible by-products) through the examination of THg and MeHg concentrations. Moreover, future work should better examine the transfer of Hg from contaminated feedstocks to these animals and determine the role Hg speciation in feedstocks plays in this transfer. Adding Hg stable isotopes to such assessments would improve our mechanistic understanding of Hg uptake, cycling, and fate within animals farmed in areas adjacent to ASGM.
Another important gap is that the effects of food preparation are not included in estimates of daily intake. Understanding of the effects of cooking on Hg and MeHg bioavailability has only recently coalesced, and is still limited to in vitro studies, which has been recently reviewed by Gong et al. (2025). The bioaccessibility of THg and MeHg vary widely between foodstuffs based on the macronutrient composition of food preparation methods (i.e., grinding vs. whole grain), and cooking methods (high temperature cooking can reduce MeHg bioaccessibility) (Gong et al., 2025). With this considered, it is essential that there be a greater focus of research into the effects of meal composition and preparation and cooking methods on Hg concentrations, speciation, and bioavailability in edible crop parts and livestock and poultry meats and eggs/dairy. This is particularly so for areas impacted by ASGM activities due to greater potential Hg exposure via contaminated foods.
Bridging these barriers will require multidisciplinary approaches involving collaboration with mine stakeholders, community leaders and engaged citizens, and both local and international scientists to conduct safe and effective site assays that effectively address the critical knowledge gaps outlined in this work. As highlighted by Moreno-Brush et al. (2020), we stress the importance of international collaborations between scientists in areas directly impacted by ASGM that possess key local partnerships and knowledges of geographies, customs, and cultures, and those from the Global North with access to greater funding opportunities and advanced methodologies (i.e., Hg stable isotope instrumentation, global fate and transport models) critical to generating the scientific robustness and impact needed to assess the impacts of ASGM Hg use on terrestrial agricultural communities. Equally vital is also ensuring knowledge translation to impacted communities post-research by promoting respectful engagement, avoiding exploitation (parachute/colonial science), and fostering lasting collaborations (Kukkonen and Cooper, 2019). The production of knowledge alone should not be the sole motivator in such efforts. Growth of ASGM is driven by demand for gold in the Global North and rapidly developing economies in Asia (Verbrugge and Geenen, 2020; Prescott et al., 2022); hence, there is responsibility that this global issue (and its impacts) requires global solutions.
No data sets were used in this article.
DSM conceptualized the review and wrote the draft of Sects. 3.1, 3.3, and 4 and rewrote sections 1 and 2 for integration in publication. EOE drafted sections 1 and 2, which were originally from EOE’s MSc thesis chapter. RJS drafted Sect. 3.2. All authors contributed to manuscript reviews and fine tuning.
At least one of the (co-)authors is a member of the editorial board of Biogeosciences. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
As noted in Fig. 1 caption, we acknowledge the use of ChatGPT (OpenAI) to create the plant and plant tissue images. However, the figure text, labels, and arrangement was completed by co-authors.
We acknowledge an internal, alumni-based, graduate scholarship/award (Maria Nathanson/IAMGOLD Fund: 50540) and DSM’s Research Initiation Grant, both from Queen's University, for funding this work.
This paper was edited by Johannes Bieser and reviewed by Jan Gacnik and one anonymous referee.
Abdulmalik, Z., Shittu, M., Adamu, S., Ambali, S. F., and Oyeyemi, B. F.: Assessment of blood mercury, immune response, heat shock and oxidative stress marker in free-ranging chicken (Gallus Gallus domesticus) from gold mining areas in Zamfara State, Nigeria, J. Hazard. Mater. Adv., 8, 100176, https://doi.org/10.1016/j.hazadv.2022.100176, 2022.
Achina-Obeng, R. and Aram, S. A.: Informal artisanal and small-scale gold mining (ASGM) in Ghana: Assessing environmental impacts, reasons for engagement, and mitigation strategies, Resour. Policy, 78, 102907, https://doi.org/10.1016/j.resourpol.2022.102907, 2022.
Adator, S. W., Wu, Q., Lambongang, M., Otoo, S. L., Bosah, C. P., and Nimako, K. O.: Farmers' perception of the impact of gold mining on shrinking agricultural land and their livelihood in the Asutifi-North District, Resour. Policy, 81, 103379, https://doi.org/10.1016/j.resourpol.2023.103379, 2023.
Addai-Arhin, S., Novirsa, R., Jeong, H. H., Phan, Q. D., Hirota, N., Ishibashi, Y., Shiratsuchi, H., and Arizono, K.: Potential human health risks of mercury-contaminated cassavas – Preliminary studies, Fundam. Toxicol. Sci., 9, 61–69, https://doi.org/10.2131/fts.9.61, 2022a.
Addai-Arhin, S., Novirsa, R., Jeong, H. H., Phan, Q. D., Hirota, N., Ishibashi, Y., Shiratsuchi, H., and Arizono, K.: The human health risks assessment of mercury in soils and plantains from farms in selected artisanal and small-scale gold mining communities around Obuasi, Ghana, J. Appl. Toxicol., 42, 258–273, https://doi.org/10.1002/jat.4209, 2022b.
Addai-Arhin, S., Novirsa, R., Jeong, H. H., Phan, Q. D., Hirota, N., Ishibashi, Y., Shiratsuchi, H., and Arizono, K.: Mercury waste from artisanal and small-scale gold mining facilities: a risk to farm ecosystems – a case study of Obuasi, Ghana, Environ. Sci. Pollut. Res., 30, 4293–4308, https://doi.org/10.1007/s11356-022-22456-4, 2023.
Adjorlolo-Gasokpoh, A., Golow, A. A., and Kambo-Dorsa, J.: Mercury in the surface soil and cassava, Manihot esculenta (flesh, leaves and peel) near goldmines at Bogoso and Prestea, Ghana, Bull. Environ. Contam. Toxicol., 89, 1106–1110, https://doi.org/10.1007/s00128-012-0849-7, 2012.
Adranyi, E., Stringer, L. C., and Altink, H.: The impacts of artisanal and small-scale gold mining on rural livelihood trajectories: Insights from Ghana, Extr. Ind. Soc., 14, 101273, https://doi.org/10.1016/j.exis.2023.101273, 2023.
Adranyi, E., Stringer, L. C., and Altink, H.: Joined-up governance for more complementary interactions between expanding artisanal small-scale gold mining and agriculture: Insights from Ghana, PLoS One, 19, e0298392, https://doi.org/10.1371/journal.pone.0298392, 2024.
Aendo, P., Mingkhwan, R., Senachai, K., Santativongchai, P., Thiendedsakul, P., and Tulayakul, P.: Health significant alarms of toxic carcinogenic risk consumption of blood meal metals contamination in poultry at a gold mining neighborhood, northern Thailand, Environ. Geochem. Health, 44, 783–797, https://doi.org/10.1007/s10653-021-00971-6, 2022.
Ahiamadjie, H., Serfor-Armah, Y., Tandoh, J., Gyampo, O., Ofosu, F., Dampare, S., Adotey, D., and Nyarko, B.: Evaluation of trace elements contents in staple foodstuffs from the gold mining areas in southwestern part of Ghana using neutron activation analysis, J. Radioanal. Nucl. Chem., 288, 653–661, https://doi.org/10.1007/s10967-011-0979-0, 2011.
Andersson, M. E., Gårdfeldt, K., Wängberg, I., and Strömberg, D.: Determination of Henry's law constant for elemental mercury, Chemosphere, 73, 587–592, https://doi.org/10.1016/j.chemosphere.2008.05.067, 2008.
Ao, M., Xu, X., Wu, Y., Zhang, C., Meng, B., Shang, L., Liang, L., Qiu, R., Wang, S., Qian, X., Zhao, L., and Qiu, G.: Newly deposited atmospheric mercury in a simulated rice ecosystem in an active mercury mining region: High loading, accumulation, and availability, Chemosphere, 238, 124630, https://doi.org/10.1016/j.chemosphere.2019.124630, 2020.
Appleton, J. D., Weeks, J. M., Calvez, J. P. S., and Beinhoff, C.: Impacts of mercury contaminated mining waste on soil quality, crops, bivalves, and fish in the Naboc River area, Mindanao, Philippines, Sci. Total Environ., 354, 198–211, https://doi.org/10.1016/j.scitotenv.2005.01.042, 2006.
Ariya, P. A., Amyot, M., Dastoor, A., Deeds, D., Feinberg, A., Kos, G., Poulain, A., Ryjkov, A., Semeniuk, K., Subir, M., and Toyota, K.: Mercury physicochemical and biogeochemical transformation in the atmosphere and at atmospheric interfaces: A review and future directions, Chem. Rev., 115, 3760–3802, https://doi.org/10.1021/cr500667e, 2015.
Arrazy, S., Addai-Arhin, S., Jeong, H., Novirsa, R., Wispriyono, B., Agusa, T., Ishibashi, Y., and Kobayashi, J.: Spatial distribution and human health risks of mercury in the gold mining area of Mandailing Natal District, Indonesia, Environ. Monit. Contam. Res., 3, 33–42, https://doi.org/10.5985/emcr.20230003, 2023.
Arrazy, S., Sone Soe, P., Novirsa, R., Wispriyono, B., Agusa, T., Ishibashi, Y., and Kobayashi, J.: Critical Review of Mercury Polluted Area in Indonesia, J. Environ. Saf., 15, 47–62, https://doi.org/10.11162/daikankyo.E23RV0801, 2024.
Aslam, M. W., Meng, B., Abdelhafiz, M. A., Liu, J., and Feng, X.: Unravelling the interactive effect of soil and atmospheric mercury influencing mercury distribution and accumulation in the soil-rice system, Sci. Total Environ., 803, 149967, https://doi.org/10.1016/j.scitotenv.2021.149967, 2022.
Bagley, M. P., Schwartz, R. A., and Lambert, W. C.: Hyperplastic reaction developing within a tattoo, Arch. Dermatol., 123, 1559–1560, https://doi.org/10.1001/archderm.1987.01660350172036, 1987.
Barkay, T. and Gu, B.: Demethylation-the other side of the mercury methylation coin: A critical review, ACS Environ. Au, acsenvironau.1c00022, https://doi.org/10.1021/acsenvironau.1c00022, 2021.
Barkay, T. and Wagner-Döbler, I.: Microbial transformations of Mercury: Potentials, challenges, and achievements in controlling mercury toxicity in the environment, Adv. Appl. Microbiol., 57, 1–52, https://doi.org/10.1016/s0065-2164(05)57001-1, 2005.
Barocas, A., Vega, C., Pardo, A. A., Flores, J. M. A., Fernandez, L., Groenendijk, J., Pisconte, J., Macdonald, D. W. and Swaisgood, R. R.: Local intensity of artisanal gold mining drives mercury accumulation in neotropical oxbow lake fishes, Sci. Tot. Environ., 886, 164024, https://doi.org/10.1016/j.scitotenv.2023.164024, 2023.
Basri, Sakakibara, M., Sera, K., and Kurniawan, I. A.: Mercury contamination of cattle in artisanal and small-scale gold mining in bombana, Southeast Sulawesi, Indonesia, Geosciences, 7, 133, https://doi.org/10.3390/geosciences7040133, 2017.
Basri, Sakakibara, M., and Sera, K.: Mercury in soil and forage plants from artisanal and small-scale gold mining in the bombana area, Indonesia, Toxics, 8, 15, https://doi.org/10.3390/toxics8010015, 2020.
Basu, N., Bastiansz, A., Dórea, J. G., Fujimura, M., Horvat, M., Shroff, E., Weihe, P., and Zastenskaya, I.: Our evolved understanding of the human health risks of mercury, Ambio, 52, 877–896, https://doi.org/10.1007/s13280-023-01831-6, 2023.
Beauford, W., Barber, J., and Barringer, A. R.: Uptake and distribution of mercury within higher plants, Physiol. Plantarum, 39, 261–265, https://doi.org/10.1111/j.1399-3054.1977.tb01880.x, 1977.
Bergquist, B. A. and Blum, J. D.: The odds and evens of mercury isotopes: Applications of mass-dependent and mass-independent isotope fractionation, Elements, 5, 353–357, https://doi.org/10.2113/gselements.5.6.353, 2009.
Bernet Kempers, E.: Between informality and organized crime: Criminalization of small-scale mining in the Peruvian rainforest, Illegal Mining: Organized Crime, Corruption, and Ecocide in a Resource-Scarce World, 273–298, https://doi.org/10.1007/978-3-030-46327-4_10, 2020.
Bjørklund, G., Dadar, M., Mutter, J., and Aaseth, J.: The toxicology of mercury: Current research and emerging trends, Environ. Res., 159, 545–554, https://doi.org/10.1016/j.envres.2017.08.051, 2017.
Bortey-Sam, N., Nakayama, S. M., Akoto, O., Ikenaka, Y., Fobil, J. N., Baidoo, E., Mizukawa, H., and Ishizuka, M.: Accumulation of heavy metals and metalloid in foodstuffs from agricultural soils around Tarkwa area in Ghana, and associated human health risks, Int. J. Environ. Res. Public Health, 12, 8811–8827, https://doi.org/10.3390/ijerph120808811, 2015.
Bose-O'Reilly, S., McCarty, K. M., Steckling, N., and Lettmeier, B.: Mercury exposure and children's health, Curr. Probl. Pediatr. Adolesc. Health Care, 40, 186–215, https://doi.org/10.1016/j.cppeds.2010.07.002, 2010.
Bose-O'Reilly, S., Schierl, R., Nowak, D., Siebert, U., William, J. F., Owi, F. T., and Ir, Y. I.: A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia, Environ. Res., 149, 274–281, https://doi.org/10.1016/j.envres.2016.04.007, 2016.
Brüger, A., Fafilek, G., Restrepo, J. B. O., and Rojas-Mendoza, L.: On the volatilisation and decomposition of cyanide contaminations from gold mining, Sci. Total Environ., 627, 1167–1173, https://doi.org/10.1016/j.scitotenv.2018.01.320, 2018.
Bugmann, A., Brugger, F., Zongo, T., and Van Der Merwe, A.: “Doing ASGM without mercury is like trying to make omelets without eggs”. Understanding the persistence of mercury use among artisanal gold miners in Burkina Faso, Environ. Sci. Policy, 133, 87–97, https://doi.org/10.1016/j.envsci.2022.03.009, 2022.
Carpi, A.: Mercury from combustion sources: A review of the chemical species emitted and their transport in the atmosphere, Water Air Soil Pollut., 98, 241–254, https://doi.org/10.1007/bf02047037, 1997.
Carrasco-Gil, S., Siebner, H., LeDuc, D. L., Webb, S. M., Millán, R., Andrews, J. C., and Hernández, L. E.: Mercury localization and speciation in plants grown hydroponically or in a natural environment, Environ. Sci. Technol., 47, 3082–3090, https://doi.org/10.1021/es303310t, 2013.
Casagrande, G. C. R., Franco, D. N. D. M., Moreno, M. I. C., de Andrade, E. A., Battirola, L. D., and de Andrade, R. L. T.: Assessment of atmospheric mercury deposition in the vicinity of artisanal and small-scale gold mines using Glycine max as bioindicators, Water Air Soil Poll., 231, 551, https://doi.org/10.1007/s11270-020-04918-y, 2020.
Castilhos, Z. C., Rodrigues-Filho, S., César, R., De Castro Rodrigues, A. P., Villas-Bôas, R., De Jesus, I., Lima, M., Faial, K. R. F., Miranda, A. M. M., Da Silva Brabo, E., Beinhoff, C., and De Oliveira Santos, E. C.: Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon, Environ. Sci. Pollut. Res., 22, 11255–11264, https://doi.org/10.1007/s11356-015-4340-y, 2015.
Cheng, Z., Wang, H.-S., Du, J., Sthiannopkao, S., Xing, G.-H., Kim, K.-W., Yasin, M. S. M., Hashim, J. H., and Wong, M.-H.: Dietary exposure and risk assessment of mercury via total diet study in Cambodia, Chemosphere, 92, 143–149, https://doi.org/10.1016/j.chemosphere.2013.02.025, 2013.
Cho, K. H., Lee, J. J., and Park, C. Y.: Liberation of gold using Microwave-Nitric acid leaching and Separation-Recovery of native gold by Hydro-Separation, Minerals, 10, 327, https://doi.org/10.3390/min10040327, 2020.
Clarkson, T. W. and Magos, L.: The toxicology of Mercury and its chemical compounds, Crit. Rev. Toxicol., 36, 609–662, https://doi.org/10.1080/10408440600845619, 2006.
Clarkson, T. W., Magos, L., and Myers, G. J.: The toxicology of Mercury – current exposures and clinical manifestations, N. Engl. J. Med., 349, 1731–1737, https://doi.org/10.1056/nejmra022471, 2003.
Compeau, G. C. and Bartha, R.: Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment, Appl. Environ. Microbiol., 50, 498–502, https://doi.org/10.1128/aem.50.2.498-502.1985, 1985.
Converse, A. D., Riscassi, A. L., and Scanlon, T. M.: Seasonal variability in gaseous mercury fluxes measured in a high-elevation meadow, Atmos. Environ., 44, 2176–2185, https://doi.org/10.1016/j.atmosenv.2010.03.024, 2010.
Cordy, P., Veiga, M. M., Salih, I., Al-Saadi, S., Console, S., García, O., Mesa, L. A., Velásquez-López, P. C., and Roeser, M.: Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world's highest per capita mercury pollution, Sci. Total Environ., 410–411, 154–160, https://doi.org/10.1016/j.scitotenv.2011.09.006, 2011.
Cordy, P., Veiga, M. M., Crawford, B., García, O., González, V. H., Moraga, D., Roeser, M., and Wip, D.: Characterization, mapping, and mitigation of mercury vapour emissions from artisanal mining gold shops, Environ. Res., 125, 82–91, https://doi.org/10.1016/j.envres.2012.10.015, 2013.
Cozzolino, V., De Martino, A., Nebbioso, A., Di Meo, V., Salluzzo, A., and Piccolo, A.: Plant tolerance to mercury in a contaminated soil is enhanced by the combined effects of humic matter addition and inoculation with arbuscular mycorrhizal fungi, Environ. Sci. Pollut. Res., 23, 11312–11322, https://doi.org/10.1007/s11356-016-6337-6, 2016.
Crout, N. M. J., Beresford, N. A., Dawson, J. M., Soar, J., and Mayes, R. W.: The transfer of 73As, 109Cd and 203Hg to the milk and tissues of dairy cattle, J. Agric. Sci., 142, 203–212, https://doi.org/10.1017/S0021859604004186, 2004.
da Silva, H. A. M. and Guimarães, J. R. D.: Mercury cyanide complexes and their relevance as environmental contaminants, Chemosphere, 141054, https://doi.org/10.1016/j.chemosphere.2023.141054, 2024.
Dastoor, A., Angot, H., Bieser, J., Christensen, J. H., Douglas, T. A., Heimbürger-Boavida, L.-E., Jiskra, M., Mason, R. P., McLagan, D. S., Obrist, D., Outridge, P. M., Petrova, M. V., Ryjkov, A., St. Pierre, K. A., Schartup, A. T., Soeresen, A. L., Toyota, K., Travnikov, O., Wilson, S. J., and Zdanowicz, C.: Arctic mercury cycling, Nat. Rev. Earth Environ., 3, 270–286, https://doi.org/10.1038/s43017-022-00269-w, 2022.
Dastoor, A., Angot, H., Bieser, J., Brocza, F., Edwards, B., Feinberg, A., Feng, X., Geyman, B., Gournia, C., He, Y., Hedgecock, I. M., Ilyin, I., Kirk, J., Lin, C.-J., Lehnherr, I., Mason, R., McLagan, D., Muntean, M., Rafaj, P., Roy, E. M., Ryjkov, A., Selin, N. E., De Simone, F., Soerensen, A. L., Steenhuisen, F., Travnikov, O., Wang, S., Wang, X., Wilson, S., Wu, R., Wu, Q., Zhang, Y., Zhou, J., Zhu, W., and Zolkos, S.: The Multi-Compartment Hg Modeling and Analysis Project (MCHgMAP): mercury modeling to support international environmental policy, Geosci. Model Dev., 18, 2747–2860, https://doi.org/10.5194/gmd-18-2747-2025, 2025.
Denzler, B., Eugster, W., Bogdal, C., Bishop, K., Buchmann, N., Hungerbühler, K., and Osterwalder, S.: Uptake of gaseous elemental mercury by a rainforest: Insights from a tropical glasshouse used as a dynamic flux chamber, Environ. Sci. Technol., 59, 18675–18686, https://doi.org/10.1021/acs.est.5c05823, 2025.
Diaz, F. A., Katz, L. E., and Lawler, D. F.: Mercury pollution in Colombia: challenges to reduce the use of mercury in artisanal and small-scale gold mining in the light of the Minamata Convention, Water Int., 45, 730–745, https://doi.org/10.1080/02508060.2020.1845936, 2020.
Donkor, P., Siabi, E. K., Frimpong, K., Frimpong, P. T., Mensah, S. K., Vuu, C., Siabi, E. S., Nyantayki, E. K., Agariga, F., Atta-Darkwa, T., and Mensah, J. K.: Impacts of illegal Artisanal and small-scale gold mining on livelihoods in cocoa farming communities: A case of Amansie West District, Ghana, Resour. Policy, 91, 104879, https://doi.org/10.1016/j.resourpol.2024.104879, 2024.
dos Santos-Lima, C., de Souza Mourão, D., de Carvalho, C. F., Souza-Marques, B., Vega, C. M., Gonçalves, R. A., Argollo, N., Menezes-Filho, J. A., Abreu, N., and de Souza Hacon, S.: Neuropsychological effects of mercury exposure in children and adolescents of the Amazon Region, Brazil, Neurotoxicol., 79, https://doi.org/10.1016/j.neuro.2020.04.004, 48–57, 2020.
Dossou Etui, I. M., Stylo, M., Davis, K., Evers, D. C., Vera, I. S., Wood, C., and Burton, M. E.: Artisanal and small-scale gold mining and biodiversity: a global literature review, Ecotoxicol., 1–21, https://doi.org/10.1007/s10646-024-02748-w, 2024.
Driscoll, C. T., Mason, R. P., Chan, H. M., Jacob, D. J., and Pirrone, N.: Mercury as a global pollutant: Sources, pathways, and effects, Environ. Sci. Technol., 47, 4967–4983, https://doi.org/10.1021/es305071v, 2013.
Du, S. H. and Fang, S. C.: Catalase activity of C3 and C4 species and its relationship to mercury vapor uptake, Environ. Exp. Bot., 23, 347–353, https://doi.org/10.1016/0098-8472(83)90009-6, 1983.
Durnford, D., Dastoor, A., Figueras-Nieto, D., and Ryjkov, A.: Long range transport of mercury to the Arctic and across Canada, Atmos. Chem. Phys., 10, 6063–6086, https://doi.org/10.5194/acp-10-6063-2010, 2010.
Eboigbe, E. O., Veerasamy, N., Odukoya, A. M., Anene, N. C., Sonke, J. E., Sagisaka Méndez, S., and McLagan, D. S.: Mercury contamination in staple crops impacted by Artisanal Small-scale Gold Mining (ASGM): Stable Hg isotopes demonstrate dominance of atmospheric uptake pathway for Hg in crops, Biogeosciences, 22, 5591–5605, https://doi.org/10.5194/bg-22-5591-2025, 2025.
Egler, S. G., Rodrigues-Filho, S., Villas-Bôas, R. C., and Beinhoff, C.: Evaluation of mercury pollution in cultivated and wild plants from two small communities of the Tapajós gold mining reserve, Pará State, Brazil, Sci. Total Environ., 368, 424–433, https://doi.org/10.1016/j.scitotenv.2005.09.037, 2006.
Ellis, E. C., Klein Goldewijk, K., Siebert, S., Lightman, D., and Ramankutty, N.: Anthropogenic transformation of the biomes, 1700 to 2000, Glob. Ecol. Biogeogr., 19, 589–606, https://doi.org/10.1111/j.1466-8238.2010.00540.x, 2010.
Esdaile, L. J. and Chalker, J. M.: The mercury problem in artisanal and Small-Scale gold mining, Chem. Eur. J., 24, 6905–6916, https://doi.org/10.1002/chem.201704840, 2018.
Essumang, D. K., Dodoo, D. K., Obiri, S. and Yaney, J. Y.: Arsenic, cadmium, and mercury in cocoyam (Xanthosoma sagititolium) and watercocoyam (Colocasia esculenta) in Tarkwa a mining community, Bull. Environ. Contam. Toxicol., 79, 377–379, https://doi.org/10.1007/s00128-007-9244-1, 2007.
Fantozzi, L., Ferrara, R., Dini, F., Tamburello, L., Pirrone, N., and Sprovieri, F.: Study on the reduction of atmospheric mercury emissions from mine waste enriched soils through native grass cover in the Mt. Amiata region of Italy, Environ. Res., 125, 69–74, https://doi.org/10.1016/j.envres.2013.02.004, 2013.
Finster, M. E., Raymond, M. R., Scofield, M. A., and Smith, K. P.: Mercury-impacted scrap metal: Source and nature of the Mercury, J. Environ. Manage., 161, 303–308, https://doi.org/10.1016/j.jenvman.2015.05.041, 2015.
Fitzgerald, W. F. and Lamborg, C. H.: Geochemistry of Mercury in the environment, in: Environmental Geochemistry, edited by: Sherwood Lollar, B., Elsevier, Oxford (UK), 107–148, https://doi.org/10.1016/b0-08-043751-6/09048-4, 2005.
Fitzgerald, W. F., Lamborg, C. H., and Hammerschmidt, C. R.: Marine biogeochemical cycling cycling of Mercury, Chem. Rev., 107, 641–662, https://doi.org/10.1021/cr050353m, 2007.
Fritsche, J., Obrist, D., Zeeman, M. J., Conen, F., Eugster, W., and Alewell, C.: Elemental mercury fluxes over a sub-alpine grassland determined with two micrometeorological methods, Atmos. Environ., 42, 2922–2933, https://doi.org/10.1016/j.atmosenv.2007.12.055, 2008.
Fu, X., Zhu, W., Zhang, H., Sommar, J., Yu, B., Yang, X., Wang, X., Lin, C.-J., and Feng, X.: Depletion of atmospheric gaseous elemental mercury by plant uptake at Mt. Changbai, Northeast China, Atmos. Chem. Phys., 16, 12861–12873, https://doi.org/10.5194/acp-16-12861-2016, 2016.
Fu, X., Zhang, H., Liu, C., Zhang, H., Lin, C. J., and Feng, X.: Significant seasonal variations in isotopic composition of atmospheric total gaseous mercury at forest sites in China caused by vegetation and mercury sources, Environ. Sci. Technol., 53, 13748–13756, https://doi.org/10.1021/acs.est.9b05016, 2019.
Gaffney, J. S. and Marley, N. A.: In-depth review of atmospheric mercury: sources, transformations, and potential sinks, Energy Emission Control. Technol., 2, 1–21, https://doi.org/10.2147/EECT.S37038, 2014.
Gačnik, J. and Gustin, M. S.: Tree rings as historical archives of atmospheric mercury: A critical review, Sci. Total Environ., 165562, https://doi.org/10.1016/j.scitotenv.2023.165562, 2023.
Gallorini, A. and Loizeau, J. L.: Mercury methylation in oxic aquatic macro-environments: a review, J. Limnol., 80, 2007, https://doi.org/10.4081/jlimnol.2021.2007, 2021.
Gerson, J. R., Szponar, N., Zambrano, A. A., Bergquist, B., Broadbent, E., Driscoll, C. T., Erkenswick, G., Evers, D. C., Fernandez, L. E., Hsu-Kim, H., and Inga, G.: Amazon forests capture high levels of atmospheric mercury pollution from artisanal gold mining, Nature Comm., 13, 559, https://doi.org/10.1038/s41467-022-27997-3, 2022.
Gilbert, D. and Albert, O. B.: Illegal small-scale gold mining in Ghana: A threat to food security, J. Food Secur., 4, 112–119, https://pubs.sciepub.com/jfs/4/5/2/ (last access: 9 July 2024), 2016.
Giron, O. D., Basa, J. E., Distor, K. S. E., and Pusing, K. G. N.: Mercury levels in Oryza sativa cultivated in Philippine small-scale mining villages, Int. J. Environ. Stud., 74, 230–239, https://doi.org/10.1080/00207233.2016.1254959, 2017.
Golow, A. A. and Adzei, E. A.: Mercury in surface soil and cassava crop near an alluvial goldmine at Dunkwa-on-Offin, Ghana, Bull. Environ. Contam. Toxicol., 69, 228–235, https://doi.org/10.1007/s00128-002-0051-4, 2002.
Gong, Y., Li, C., He, F., Ge, F., Ju, Y., Zhong, H., and Li, W.: Comprehensive review on in vitro bioaccessibility of mercury in various foodstuffs, J. Hazard. Mater., 138136, https://doi.org/10.1016/j.jhazmat.2025.138136, 2025.
Graydon, J. A., St. Louis, V. L., Hintelmann, H., Lindberg, S. E., Sandilands, K. A., Rudd, J. W., Kelly, C. A., Tate, M. T., Krabbenhoft, D. P., and Lehnherr, I.: Investigation of uptake and retention of atmospheric Hg (II) by boreal forest plants using stable Hg isotopes, Environ. Sci. Technol., 43, 4960–4966, https://doi.org/10.1021/es900357s, 2009.
Grégoire, D. S. and Poulain, A. J.: A physiological role for HgII during phototrophic growth, Nat. Geosci., 9, 121–125, https://doi.org/10.1038/ngeo2629, 2016.
Guzzi, G. and La Porta, C. A. M.: Molecular mechanisms triggered by Mercury, Toxicology, 244, 1–12, https://doi.org/10.1016/j.tox.2007.11.002, 2008.
Gworek, B., Dmuchowski, W., and Baczewska-Dąbrowska, A. H.: Mercury in the Terrestrial Environment: A Review, Environ. Sci. Eur., 32, 128, https://doi.org/10.1186/s12302-020-00401-x, 2020.
Ha, E., Basu, N., Bose-O'Reilly, S., Dórea, J. G., McSorley, E., Sakamoto, M., and Chan, H. M.: Current progress on understanding the impact of Mercury on human health, Environ. Res., 152, 419–433, https://doi.org/10.1016/j.envres.2016.06.042, 2017.
Hamelin, S., Planas, D., and Amyot, M.: Mercury methylation and demethylation by periphyton biofilms and their host in a fluvial wetland of the St. Lawrence River (QC, Canada), Sci. Total Environ., 512–513, 464–471, https://doi.org/10.1016/j.scitotenv.2015.01.040, 2015.
Hao, Y.-Y., Zhu, Y.-J., Yan, R.-Q., Gu, B., Zhou, X.-Q., Wei, R.-R., Wang, C., Feng, J., Huang, Q., and Liu, Y.-R.: Important roles of thiols in methylmercury uptake and translocation by rice plants, Environ. Sci., 9, https://doi.org/10.1021/acs.est.2c00169, 2022.
Hardy, A. D., Sutherland, H. H., Vaishnav, R., and Worthing, M. A.: A report on the composition of Mercurials used in traditional medicines in Oman, J. Ethnopharmacol., 49, 17–22, https://doi.org/10.1016/0378-8741(95)01296-6, 1995.
Hedgecock, I. M., De Simone, F., Carbone, F., and Pirrone, N.: Modelling the Fate of Mercury Emissions from Artisanal and Small Scale Gold Mining, EGUsphere [preprint], https://doi.org/10.5194/egusphere-2024-861, 2024.
Hentschel, T., Hruschka, F., and Priester, M.: Mining, minerals and sustainable development Project: Global report on artisanal and small-scale mining, International Institute for Environment and Development (IIED), London, UK, 70 pp., https://intranetua.uantof.cl/crea/cguerra/pdffiles/otros/070_globalasm.pdf (last access: 10 September 2024), 2002.
Hilson, G.: Abatement of mercury pollution in the small-scale gold mining industry: Restructuring the policy and research agendas, Sci. Total Environ., 362, 1–14, https://doi.org/10.1016/j.scitotenv.2005.09.065, 2006.
Hilson, G.: “Fair trade gold”: Antecedents, prospects and challenges, Geoforum, 39, 386–400, https://doi.org/10.1016/j.geoforum.2007.09.003, 2008.
Hindersah, R., Risamasu, R., Kalay, A. M., Dewi, T., and Makatita, I.: Mercury contamination in soil, tailing and plants on agricultural fields near closed gold mine in Buru Island, Maluku, J. Degraded Min. Land Manage., 5, 1027–1034, https://doi.org/10.15243/jdmlm.2018.052.1027, 2018.
Hintelmann, H., Keppel-Jones, K., and Evans, R. D.: Constants of mercury methylation and demethylation rates in sediments and comparison of tracer and ambient mercury availability, Environ. Toxicol. Chem., 19, 2204–2211, https://doi.org/10.1002/etc.5620190909, 2000.
Hinton, J., Veiga, M. M., and Veiga, A.: Clean artisanal gold mining: a utopian approach?, J. Cleaner Prod., 11, 99–115, https://doi.org/10.1016/s0959-6526(02)00031-8, 2003.
Holmes, C. D., Jacob, D. J., Corbitt, E. S., Mao, J., Yang, X., Talbot, R., and Slemr, F.: Global atmospheric model for mercury including oxidation by bromine atoms, Atmos. Chem. Phys., 10, 12037–12057, https://doi.org/10.5194/acp-10-12037-2010, 2010.
Horowitz, H. M., Jacob, D. J., Zhang, Y., Dibble, T. S., Slemr, F., Amos, H. M., Schmidt, J. A., Corbitt, E. S., Marais, E. A., and Sunderland, E. M.: A new mechanism for atmospheric mercury redox chemistry: implications for the global mercury budget, Atmos. Chem. Phys., 17, 6353–6371, https://doi.org/10.5194/acp-17-6353-2017, 2017.
Hosonuma, N., Herold, M., De Sy, V., De Fries, R. S., Brockhaus, M., Verchot, L., Angelsen, A., and Romijn, E.: An assessment of deforestation and forest degradation drivers in developing countries, Environ. Res. Lett., 7, 044009, https://doi.org/10.1088/1748-9326/7/4/044009, 2012.
Huang, R., Zhou, Q., Meng, B., Zhang, S., Jiang, T., Yin, D., Li, B., Wang, C., Gao, X., Liu, J., Huang, J.-H., and Feng, X.: Coupling of mercury contamination and carbon emissions in rice paddies: Methylmercury dynamics versus CO2 and CH4 emissions, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.5c01099, 2025.
Jasinski, S. M.: The materials flow of mercury in the United States, Resour. Conserv. Recycl., 15, 145–179, https://doi.org/10.1016/0921-3449(95)00032-1, 1995.
Jiang, G.-B., Shi, J.-B., and Feng, X.-B.: Mercury pollution in China, Environ. Sci. Technol., 40, 3672–3678, https://doi.org/10.1021/es062707c, 2006.
Jiskra, M., Sonke, J. E., Obrist, D., Bieser, J., Ebinghaus, R., Myhre, C. L., Pfaffhuber, K. A., Wängberg, I., Kyllönen, K., Worthy, D. Martin, L. G., Labuschagne, C., Mkololo, T., Ramonet, M., Magand, O., and Dommergue, A.: A vegetation control on seasonal variations in global atmospheric mercury concentrations, Nat. Geosci., 11, 244–250, https://doi.org/10.1038/s41561-018-0078-8, 2018.
Jønsson, J. B., Charles, E., and Kalvig, P.: Toxic mercury versus appropriate technology: Artisanal gold miners' retort aversion, Resour. Policy, 38, 60–67, https://doi.org/10.1016/j.resourpol.2012.09.001, 2013.
Kern, J. K., Geier, D. A., Sykes, L. K., Haley, B. E., and Geier, M. R.: The relationship between Mercury and autism: A comprehensive review and discussion, J. Trace Elem. Med. Biol., 37, 8–24, https://doi.org/10.1016/j.jtemb.2016.06.002, 2016.
Kiefer, A. M., Drace, K., Seney, C. S., and Veiga, M. M.: Challenges associated with using retorts to limit mercury exposure in artisanal and small-scale gold mining: case studies from Mozambique, Ecuador, and Guyana, in: Trace Materials in Air, Soil, and Water, American Chemical Society, Washington (USA), 51–77, https://doi.org/10.1021/bk-2015-1210.ch003, 2015.
Kinimo, K. C., Yao, K. M., Marcotte, S., Kouassi, N. L. B., and Trokourey, A.: Trace metal(loid)s contamination in paddy rice (Oryza sativa L.) from wetlands near two gold mines in Côte d'Ivoire and health risk assessment, Environ. Sci. Pollut. Res., 28, 22779–22788, https://doi.org/10.1007/s11356-021-12360-8, 2021.
Klasing, K. C.: Poultry nutrition: a comparative approach, J. Appl. Poult. Res., 14, 426–436, https://doi.org/10.1093/japr/14.2.426, 2005.
Kögel-Knabner, I., Amelung, W., Cao, Z., Fiedler, S., Frenzel, P., Jahn, R., Kalbitz, K., Kölbl, A., and Schloter, M.: Biogeochemistry of paddy soils, Geoderma, 157, 1–14, https://doi.org/10.1016/j.geoderma.2010.03.009, 2010.
Krisnayanti, B. D., Anderson, C. W., Utomo, W. H., Feng, X., Handayanto, E., Mudarisna, N., and Ikram, H.: Assessment of environmental mercury discharge at a four-year-old artisanal gold mining area on Lombok Island, Indonesia, J. Environ. Monit., 14, 2598–2607, https://doi.org/10.1039/C2EM30515A, 2012.
Kritee, K., Blum, J. D., Johnson, M. W., Bergquist, B. A., and Barkay, T.: Mercury stable isotope fractionation during reduction of Hg(II) to Hg(0) by Mercury Resistant Microorganisms, Environ. Sci. Technol., 41, 1889–1895, https://doi.org/10.1021/es062019t, 2007.
Kukkonen, T. and Cooper, A.: An arts-based knowledge translation (ABKT) planning framework for researchers, Evid. Policy J. Res. Debate Pract., 15, 293–311, https://doi.org/10.1332/174426417x15006249072134, 2019.
Laacouri, A., Nater, E. A., and Kolka, R. K.: Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, USA, Environ. Sci. Technol., 47, 10462–10470, https://doi.org/10.1021/es401357z, 2013.
Lei, P., Yu, R., Kong, Y., Bertilsson, S., Tsui, M. T., Jiang, T., Zhao, J., Liu, Y., Rinklebe, J., and Zhong, H.: Properly interpret metabolic inhibition results to identify primary mercury methylating microbes, Crit. Rev. Environ. Sci. Technol., 53, 1757–1773, https://doi.org/10.1080/10643389.2023.2183072, 2023.
Li, Y. and Cai, Y.: Progress in the study of mercury methylation and demethylation in aquatic environments, Chin. Sci. Bull., 58, 177–185, https://doi.org/10.1007/s11434-012-5416-4, 2012.
Li, Y., Zhao, J., Zhang, B., Liu, Y., Xu, X., Li, Y.-F., Li, B., Gao, Y., and Chai, Z.: The influence of iron plaque on the absorption, translocation and transformation of mercury in rice (Oryza sativa L.) seedlings exposed to different mercury species, Plant Soil, https://doi.org/10.1007/s11104-015-2627-x, 2015.
Lindberg, S. E., Bullock, R., Ebinghaus, R., Engstrom, D., Feng, X., Fitzgerald, W., Pirrone, N., Prestbo, E., and Seigneur, C.: A synthesis of progress and uncertainties in attributing the sources of mercury in deposition, Ambio, 36, 19–33, https://doi.org/10.1579/0044-7447(2007)36[19:asopau]2.0.co;2, 2007.
Liu, J., Meng, B., Poulain, A. J., Meng, Q., and Feng, X.: Stable isotope tracers identify sources and transformations of mercury in rice (Oryza sativa L.) growing in a mercury mining area, Fundam. Res., 1, 259–268, https://doi.org/10.1016/j.fmre.2021.04.003, 2021a.
Liu, M., Zhang, Q., Cheng, M., He, Y., Chen, L., Zhang, H., Cao, H., Shen, H., Zhang, W., Tao, S., and Wang, X.: Rice life cycle-based global mercury biotransport and human methylmercury exposure, Nat. Commun., 10, 5164, https://doi.org/10.1038/s41467-019-13221-2, 2019.
Liu, X., Wang, X., and Wang, D.: Assessment of tree-ring mercury radial translocation and age effect in Masson pine: Implications for historical atmospheric mercury reconstruction, J. Environ. Sci., 138, 266–276, https://doi.org/10.1016/j.jes.2022.10.027, 2024.
Liu, Y., Lin, C. J., Yuan, W., Lu, Z., and Feng, X.: Translocation and distribution of mercury in biomasses from subtropical forest ecosystems: Evidence from stable mercury isotopes, Acta Geochim., 40, 42–50, https://doi.org/10.1007/s11631-020-00441-3, 2021b.
Liu, Y., Liu, G., Wang, Z., Guo, Y., Yin, Y., Zhang, X., Cai, Y., and Jiang, G.: Understanding foliar accumulation of atmospheric Hg in terrestrial vegetation: Progress and challenges, Crit. Rev. Environ. Sci. Technol., 52, 4331–4352, https://doi.org/10.1080/10643389.2021.1989235, 2022.
Liu, Y.-R., Dong, J.-X., Han, L.-L., Zheng, Y.-M., and He, J.-Z.: Influence of rice straw amendment on mercury methylation and nitrification in paddy soils, Environ. Pollut., 209, 53–59, https://doi.org/10.1016/j.envpol.2015.11.023, 2016.
Liu, Y.-R., Johs, A., Bi, L., Lu, X., Hu, H.-W., Sun, D., He, J.-Z., and Gu, B.: Unraveling microbial communities associated with methylmercury production in paddy soils, Environ. Sci. Technol., 52, 13110–13118, https://doi.org/10.1021/acs.est.8b03052, 2018.
Lomonte, C., Wang, Y., Doronila, A., Gregory, D., Baker, A. J., Siegele, R., and Kolev, S. D.: Study of the spatial distribution of mercury in roots of vetiver grass (Chrysopogon zizanioides) by micro-PIXE spectrometry, Int. J. Phytoremediation, 16, 1170–1182, https://doi.org/10.1080/15226514.2013.821453, 2014.
Mallongi, A., Pataranawat, P., and Parkpian, P.: Mercury emission from artisanal buladu gold mine and its bioaccumulation in rice grains, Gorontalo Province, Indonesia, Adv. Mater. Res., 931, 744–748, https://doi.org/10.4028/www.scientific.net/AMR.931-932.744, 2014.
Malone, A., Smith, N. M., and Zeballos, E. Z.: Coexistence and conflict between artisanal mining, fishing, and farming in a Peruvian boomtown, Geoforum, 120, 142–154, https://doi.org/10.1016/j.geoforum.2021.01.012, 2021.
Malone, A., Figueroa, L., Wang, W., Smith, N. M., Ranville, J. F., Vuono, D. C., Zapata, F. D. A., Paredes, L. M., Sharp, J. O., and Bellona, C.: Transitional dynamics from mercury to cyanide-based processing in artisanal and small-scale gold mining: Social, economic, geochemical, and environmental considerations, Sci. Total Environ., 898, 165492, https://doi.org/10.1016/j.scitotenv.2023.165492, 2023.
Manceau, A., Wang, J., Rovezzi, M., Glatzel, P., and Feng, X.: Biogenesis of mercury–sulfur nanoparticles in plant leaves from atmospheric gaseous mercury, Environ. Sci. Technol., 52, 3935–3948, https://doi.org/10.1021/acs.est.7b05452, 2018.
Marchese, M. J., Gerson, J. R., Berky, A. J., Driscoll, C., Fernandez, L. E., Hsu-Kim, H., Lansdale, K. N., Letourneau, E., Montesdeoca, M., Pan, W. K., and Robie, E.: Diet choices determine mercury exposure risks for people living in gold mining regions of Peru, Environ. Res. Health, 2, 035001, https://doi.org/10.1088/2752-5309/ad3d79, 2024.
Marshall, B., Camacho, A. A., Jimenez, G., and Veiga, M. M.: Mercury challenges in Mexico: regulatory, trade and environmental impacts, Atmosphere, 12, 57, https://doi.org/10.3390/atmos12010057, 2020.
Marvin-DiPasquale, M. C. and Oremland, R. S.: Bacterial methylmercury degradation in Florida Everglades peat sediment, Environ. Sci. Technol., 32, 2556–2563, 1998.
Marvin-DiPasquale, M., Agee, J., McGowan, C., Oremland, R. S., Thomas, M., Krabbenhoft, D., and Gilmour, C. C.: Methyl-mercury degradation pathways: A comparison among three mercury-impacted ecosystems, Environ. Sci. Technol., 34, 4908–4916, https://doi.org/10.1021/es0013125, 2000.
Maurice-Bourgoin, L., Quiroga, I. L. J., Guyot, J. L., and Malm, O.: Mercury Pollution in the Upper Beni River, Ambio, 28, 302–306, https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_7/b_fdi_51-52/010019126.pdf (last access: 9 July 2024), 1999.
McLagan, D. S., Biester, H., Navrátil, T., Kraemer, S. M., and Schwab, L.: Internal tree cycling and atmospheric archiving of mercury: examination with concentration and stable isotope analyses, Biogeosciences, 19, 4415–4429, https://doi.org/10.5194/bg-19-4415-2022, 2022a.
McLagan, D. S., Schwab, L., Wiederhold, J. G., Chen, L., Pietrucha, J., Kraemer, S. M., and Biester, H.: Demystifying mercury geochemistry in contaminated soil–groundwater systems with complementary mercury stable isotope, concentration, and speciation analyses, Environ. Sci. Process. Impacts, 24, 1406–1429, https://doi.org/10.1039/D1EM00368B, 2022b.
Meng, B., Feng, X., Qiu, G., Cai, Y., Wang, D., Li, P., Shang, L., and Sommar, J.: Distribution Patterns of Inorganic Mercury and Methylmercury in Tissues of Rice (Oryza sativa L.) Plants and Possible Bioaccumulation Pathways, J. Agric. Food Chem., 58, 4951–4958, https://doi.org/10.1021/jf904557x, 2010.
Meng, B., Feng, X., Qiu, G., Anderson, C. W. N., Wang, J., and Zhao, L.: Localization and speciation of mercury in brown rice with implications for Pan-Asian public health, Environ. Sci. Technol., 48, 7974–7981, https://doi.org/10.1021/es502000d, 2014.
Mestanza-Ramón, C., Mora-Silva, D., D'Orio, G., Tapia-Segarra, E., Gaibor, I. D., Esparza Parra, J. F., Chávez Velásquez, C. R., and Straface, S.: Artisanal and Small-Scale Gold Mining (ASGM): Management and Socioenvironmental Impacts in the Northern Amazon of Ecuador, Sustainability, 14, 6854, https://doi.org/10.3390/su14116854, 2022.
Moody, K. H., Hasan, K. M., Aljic, S., Blakeman, V. M., Hicks, L. P., Loving, D. C., Moore, M. E., Hammett, B. S., Silva-González, M., Seney, C. S., and Kiefer, A. M.: Mercury emissions from Peruvian gold shops: Potential ramifications for Minamata compliance in artisanal and small-scale gold mining communities, Environ. Res., 182, 109042, https://doi.org/10.1016/j.envres.2019.109042, 2020.
Moreno-Brush, M., McLagan, D. S., and Biester, H.: Fate of mercury from artisanal and small-scale gold mining in tropical rivers: Hydrological and biogeochemical controls. A critical review, Crit. Rev. Environ. Sci. Technol., 50, 437–475, https://doi.org/10.1080/10643389.2019.1629793, 2019.
Moreno-Brush, M., McLagan, D. S., and Biester, H.: Fate of mercury from artisanal and small-scale gold mining in tropical rivers: Hydrological and biogeochemical controls, A critical review, Crit. Rev. Environ. Sci. Technol., 50, 437–475, https://doi.org/10.1080/10643389.2019.1629793, 2020.
Mowat, L. D., St. Louis, V. L., Graydon, J. A., and Lehnherr, I.: Influence of forest canopies on the deposition of methylmercury to boreal ecosystem watersheds, Environ. Sci. Technol., 45, 5178–5185, https://doi.org/10.1021/es104377y, 2011.
Munthe, J., Kindbom, K., Parsmo, R., and Yaramenka, K.: Technical Background Report to the Global Mercury Assessment 2018, United Nations Environmental Programme (UNEP), Kenya (NGA), 136 pp., https://www.unep.org/globalmercurypartnership/resources/report/technical-background-report-global-mercury-assessment-2018 (last access: 24 June 2024), 2019.
Naharro, R., Esbrí, J. M., Amorós, J. A., and Higueras, P. L.: Experimental assessment of the daily exchange of atmospheric mercury in Epipremnum aureum, Environ. Geochem. Health, 42, 3185–3198, https://doi.org/10.1007/s10653-020-00557-8, 2020.
Navrátil, T., Šimeček, M., Shanley, J. B., Rohovec, J., Hojdová, M., and Houška, J.: The history of mercury pollution near the Spolana chlor-alkali plant (Neratovice, Czech Republic) as recorded by Scots pine tree rings and other bioindicators, Sci. Total Environ., 586, 1182–1192, https://doi.org/10.1016/j.scitotenv.2017.02.112, 2017.
Nöller, R.: Cinnabar reviewed: characterization of the red pigment and its reactions, Stud. Conserv., 60, 79–87, https://doi.org/10.1179/2047058413y.0000000089, 2014.
Norrby, L. J.: Why is Mercury Liquid? or, why do relativistic effects not get into chemistry textbooks? J. Chem. Ed., 68, 110, https://doi.org/10.1021/ed068p110, 1991.
Nyanza, E. C., Dewey, D., Thomas, D. S., Davey, M., and Ngallaba, S. E.: Spatial distribution of mercury and arsenic levels in water, soil and cassava plants in a community with long history of gold mining in Tanzania, Bull. Environ. Contam. Toxicol., 93, 716–721, https://doi.org/10.1007/s00128-014-1315-5, 2014.
Novirsa, R., Dinh, Q. P., Jeong, H., Fukushima, S., Ishibashi, Y., Wispriyono, B., and Arizono, K.: The evaluation of mercury contamination in upland rice paddy field around artisanal small-scale gold mining area, Lebaksitu, Indonesia, J. Environ. Saf., 10, 119–125, 2019.
Novirsa, R., Dinh, Q. P., Jeong, H., Addai-Arhin, S., Nugraha, W. C., Hirota, N., Wispriyono, B., Ishibashi, Y., and Arizono, K.: The dietary intake of mercury from rice and human health risk in artisanal small-scale gold mining area, Indonesia, Fundam. Toxicol. Sci., 7, 215–225, https://doi.org/10.2131/fts.7.215, 2020.
Ogola, J., Mitullah, W., and Omulo, M. A.: Impact of gold mining on the environment and human health: A case study in the migori gold belt, kenya, Environ. Geochem. Health, 24, 141–157, https://doi.org/10.1023/a:1014207832471, 2002.
Osei, L., Arku, G., and Isaac, L.: “We have done nothing wrong”: Youth miners' perceptions of the environmental consequences of artisanal and small-scale mining (ASM) in Ghana, Extr. Ind. Soc., 12, 101179, https://doi.org/10.1016/j.exis.2022.101179, 2022.
Outridge, P. M., Mason, R. P., Wang, F., Guerrero, S., and Heimburger-Boavida, L. E.: Updated global and oceanic mercury budgets for the United Nations Global Mercury Assessment 2018, Environ. Sci. Technol., 52, 11466–11477, https://doi.org/10.1021/acs.est.8b01246, 2018.
Pacyna, E. G., Pacyna, J. M., Fudała, J., Strzelecka-Jastrząb, E., Hławiczka, S., and Panasiuk, D.: Mercury emissions to the atmosphere from anthropogenic sources in Europe in 2000 and their scenarios until 2020, Sci. Total Environ., 370, 147–156, https://doi.org/10.1016/j.scitotenv.2006.06.023, 2006.
Pacyna, E. G., Pacyna, J. M., Sundseth, K., Munthe, J., Kindbom, K., Wilson, S., Steenhuisen, F., and Maxson, P. A.: Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020, Atmos. Environ., 44, 2487–2499, https://doi.org/10.1016/j.atmosenv.2009.06.009, 2010.
Park, J.-D. and Zheng, W.: Human exposure and health effects of Inorganic and elemental Mercury, J. Prev. Med. Public Health, 45, 344–352, https://doi.org/10.3961/jpmph.2012.45.6.344, 2012.
Parsaei, P., Rahimi, E., and Shakerian, A.: Concentrations of cadmium, lead and mercury in raw bovine, ovine, caprine, buffalo and camel milk, Pol. J. Environ. Stud., 28, 4311, https://doi.org/10.15244/pjoes/94809, 2019.
Pataranawat, P., Parkpian, P., Polprasert, C., Delaune, R. D., and Jugsujinda, A.: Mercury emission and distribution: Potential environmental risks at a small-scale gold mining operation, Phichit Province, Thailand, J. Environ. Sci. Health A, 42, 1081–1093, https://doi.org/10.1080/10934520701418573, 2007.
Peralta-Videa, J. R., Lopez, M. L., Narayan, M., Saupe, G., and Gardea-Torresdey, J.: The biochemistry of environmental heavy metal uptake by plants: implications for the food chain, Int. J. Biochem. Cell Biol., 41, 1665–1677, https://doi.org/10.1016/j.biocel.2009.03.005, 2009.
Pfeiffer, W. C. and De Lacerda, L. D.: Mercury inputs into the Amazon Region, Brazil, Environ. Technol. Lett., 9, 325–330, https://doi.org/10.1080/09593338809384573, 1988.
Pirrone, N., Cinnirella, S., Feng, X., Finkelman, R. B., Friedli, H. R., Leaner, J., Mason, R., Mukherjee, A. B., Stracher, G. B., Streets, D. G., and Telmer, K.: Global mercury emissions to the atmosphere from anthropogenic and natural sources, Atmos. Chem. Phys., 10, 5951–5964, https://doi.org/10.5194/acp-10-5951-2010, 2010.
Prescott, G. W., Baird, M., Geenen, S., Nkuba, B., Phelps, J., and Webb, E. L.: Formalizing artisanal and small-scale gold mining: A grand challenge of the Minamata Convention, One Earth, 5, 242–251, https://doi.org/10.1016/j.oneear.2022.02.005, 2022.
Qian, X., Yang, C., Xu, X., Ao, M., Xu, Z., Wu, Y., and Qiu, G.: Extremely elevated total mercury and methylmercury in forage plants in a large-scale abandoned hg mining site: a potential risk of exposure to grazing animals, Arch. Environ. Contam. Toxicol., 80, 519–530, https://doi.org/10.1007/s00244-021-00826-2, 2021.
Qin, C., Du, B., Yin, R., Meng, B., Fu, X., Li, P., Zhang, L., and Feng, X.: Isotopic fractionation and source appointment of methylmercury and inorganic mercury in a paddy ecosystem, Environ. Sci. Technol., 54, 14334–14342, https://doi.org/10.1021/acs.est.0c03341, 2020.
Qiu, G., Feng, X., Li, P., Wang, S., Li, G., Shang, L., and Fu, X.: Methylmercury accumulation in rice (Oryza sativa L.) grown at abandoned mercury mines in Guizhou, China, J. Agric. Food Chem., 56, 2465–2468, https://doi.org/10.1021/jf073391a, 2008.
Ramlan, Basir-Cyio, M., Napitupulu, M., Inoue, T., Anshary, A., Mahfudz, Isrun, Rusydi, M., Golar, Sulbadana, and Bakri, R.: Pollution and contamination level of Cu, Cd, and Hg heavy metals in soil and food crop, Int. J. Environ. Sci. Technol., 19, 1153–1164, https://doi.org/10.1007/s13762-021-03345-8, 2022.
Rea, A. W., Lindberg, S. E., and Keeler, G. J.: Assessment of dry deposition and foliar leaching of mercury and selected trace elements based on washed foliar and surrogate surfaces, Environ. Sci. Technol., 34, 2418–2425, https://doi.org/10.1021/es991305k, 2000.
Rea, A. W., Lindberg, S. E., Scherbatskoy, T. A., and Keeler, G. J.: Mercury accumulation in foliage over time in two northern mixed-hardwood forests, Water Air Soil Pollut., 133, 49–67, https://doi.org/10.1023/A:1012919731598, 2002.
Rothenberg, S. E., Feng, X., Zhou, W., Tu, M., Jin, B., and You, J.: Environment and genotype controls on mercury accumulation in rice (Oryza sativa L.) cultivated along a contamination gradient in Guizhou, China, Sci. Total Environ., 426, 272–280, https://doi.org/10.1016/j.scitotenv.2012.03.024, 2012.
Rothenberg, S. E., Windham-Myers, L., and Creswell, J. E.: Rice methylmercury exposure and mitigation: A comprehensive review, Environ. Res., 133, 407–423, https://doi.org/10.1016/j.envres.2014.03.001, 2014.
Rothenberg, S. E., Mgutshini, N. L., Bizimis, M., Johnson-Beebout, S. E., and Ramanantsoanirina, A.: Retrospective study of methylmercury and other metal(loid)s in Madagascar unpolished rice (Oryza sativa L.), Environ. Pollut., 196, 125–133, https://doi.org/10.1016/j.envpol.2014.10.002, 2015.
Rutter, A. P., Schauer, J. J., Shafer, M. M., Creswell, J. E., Olson, M. R., Robinson, M., Collins, R. M., Parman, A. M., Katzman, T. L., and Mallek, J. L.: Dry deposition of gaseous elemental mercury to plants and soils using mercury stable isotopes in a controlled environment, Atmos. Environ., 45, 848–855, https://doi.org/10.1016/j.atmosenv.2010.11.025, 2011a.
Rutter, A. P., Schauer, J. J., Shafer, M. M., Creswell, J., Olson, M. R., Clary, A., Robinson, M., Parman, A. M., and Katzman, T. L.: Climate sensitivity of gaseous elemental mercury dry deposition to plants: Impacts of temperature, light intensity, and plant species, Environ. Sci. Technol., 45, 569–575, https://doi.org/10.1021/es102687b, 2011b.
Saiz-Lopez, A., Sitkiewicz, S. P., Roca-Sanjuán, D., Oliva-Enrich, J. M., Dávalos, J. Z., Notario, R., Jiskra, M., Yang, X., Wang, F., Thackray, C. P., Sunderland, E. M., Jacob, D., Travnikov, O., Cuevas, C. A., Acuña, A. U., Rivero, D., Plane, J. M. C., Kinnison, D. E., and Sonke, J. E.: Photoreduction of gaseous oxidized mercury changes global atmospheric mercury speciation, transport and deposition, Nat. Commun., 9, 4769, https://doi.org/10.1038/s41467-018-07075-3, 2018.
Sakata, M. and Marumoto, K.: Wet and dry deposition fluxes of mercury in Japan, Atmos. Environ., 39, 3139–3146, https://doi.org/10.1016/j.atmosenv.2005.01.049, 2005.
Sanga, T. R., Maseka, K. K., Ponraj, M., Tungaraza, C., Mng'ong'o, M. E., and Mwakalapa, E. B.: Accumulation and distribution of mercury in agricultural soils, food crops and associated health risks: A case study of Shenda gold mine – Geita Tanzania, Environ. Challenges, 11, 100697, https://doi.org/10.1016/j.envc.2023.100697, 2023.
Saragih, G. S., Tapriziah, E. R., Syofyan, Y., Masitoh, S., Pandiangan, Y. S. H., and Andriantoro, A.: Mercury contamination in selected edible plants and soil from artisanal and small-scale gold mining in Sukabumi Regency, Indonesia, Makara J. Sci., 25, 4, https://doi.org/10.7454/mss.v25i4.1280, 2021.
Schettler, T.: Toxic threats to neurologic development of children, Environ. Health Perspect., 109, 813–816, https://doi.org/10.1289/ehp.01109s6813, 2001.
Schroeder, W. H. and Munthe, J.: Atmospheric Mercury – an overview, Atmos. Environ., 32, 809–822, https://doi.org/10.1016/s1352-2310(97)00293-8, 1998.
Schuster, E.: The behavior of Mercury in the soil with special emphasis on complexation and adsorption processes – A review of the literature, Water Air Soil Pollut., 56, 667–680, https://doi.org/10.1007/bf00342308, 1991.
Schwartz, F. W., Lee, S., and Darrah, T. H.: A review of health issues related to child labor and violence within artisanal and small-scale mining, https://doi.org/10.1029/2020GH000326, 2021.
Seccatore, J., Veiga, M. M., Origliasso, C., Marin, T., and De Tomi, G.: An estimation of the artisanal small-scale production of gold in the world, Sci. Total Environ., 496, 662–667, https://doi.org/10.1016/j.scitotenv.2014.05.003, 2014.
Seney, C. S., Bridges, C. C., Aljic, S., Moore, M. E., Orr, S. E., Barnes, M. C., Joshee, L., Uchakina, O. N., Bellott, B. J., McKallip, R. J., and Drace, K.: Reaction of cyanide with Hg0-contaminated gold mining tailings produces soluble mercuric cyanide complexes, Chem. Res. Toxicol., 33, 2834–2844, https://doi.org/10.1021/acs.chemrestox.0c00211, 2020.
Siwik, E. I., Campbell, L. M., and Mierle, G.: Distribution and trends of mercury in deciduous tree cores, Environ. Pollut., 158, 2067–2073, https://doi.org/10.1016/j.envpol.2010.03.002, 2010.
Ssenku, J. E., Naziriwo, B., Kutesakwe, J., Mustafa, A. S., Kayeera, D., and Tebandeke, E.: Mercury accumulation in food crops and phytoremediation potential of wild plants thriving in artisanal and small-scale gold mining areas in Uganda, Pollutants, 3, 181–196, https://doi.org/10.3390/pollutants3020014, 2023.
Streets, D. G., Devane, M. K., Lu, Z., Bond, T. C., Sunderland, E. M., and Jacob, D. J.: All-time releases of mercury to the atmosphere from human activities, Environ. Sci. Technol., 45, 10485–10491, https://doi.org/10.1021/es202765m, 2011.
Streets, D. G., Horowitz, H. M., Lü, Z., Levin, L., Thackray, C. P., and Sunderland, E. M.: Global and regional trends in mercury emissions and concentrations, 2010–2015, Atmos. Environ., 201, 417–427, https://doi.org/10.1016/j.atmosenv.2018.12.031, 2019.
Strickman, R. J. and Mitchell, C. P.: Accumulation and translocation of methylmercury and inorganic mercury in Oryza sativa: An enriched isotope tracer study, Sci. Total Environ., 574, 1415–1423, https://doi.org/10.1016/j.scitotenv.2016.08.068, 2017.
Sun, R., Jiskra, M., Amos, H. M., Zhang, Y., Sunderland, E. M., and Sonke, J. E.: Modelling the Mercury stable isotope distribution of Earth Surface Reservoirs: Implications for global HG cycling, Geochim. Cosmochim. Acta, 246, 156–173, https://doi.org/10.1016/j.gca.2018.11.036, 2019.
Sun, T., Wang, Z., Zhang, X., Niu, Z., and Chen, J.: Influences of high-level atmospheric gaseous elemental mercury on methylmercury accumulation in maize (Zea mays L.), Environ. Pollut., 265, 114890, https://doi.org/10.1016/j.envpol.2020.114890, 2020.
Swain, E. B., Jakus, P. M., Rice, G., Lupi, F., Maxson, P. A., Pacyna, J. M., Penn, A., Spiegel, S. J., and Veiga, M. M.: Socioeconomic consequences of mercury use and pollution, Ambio, 36, 45–61, https://doi.org/10.1579/0044-7447(2007)36[45:SCOMUA]2.0.CO;2, 2007.
Swenson, J. J., Carter, C. E., Domec, J., and Delgado, C. I.: Gold mining in the Peruvian Amazon: global prices, deforestation, and mercury imports, PLoS One, 6, e18875, https://doi.org/10.1371/journal.pone.0018875, 2011.
Szponar, N., Su, Y., Stupple, G., McLagan, D. S., Pilote, M., Munoz, A., Mitchell, C. P., Steffen, A., Wania, F., and Bergquist, B. A.: Applying passive air sampling and isotopic characterization to assess spatial variability of gaseous elemental mercury across Ontario, Canada, J. Geophys. Res. Atmos., 128, e2022JD037361, https://doi.org/10.1029/2022JD037361, 2023.
Szponar, N., Vega, C. M., Gerson, J., McLagan, D. S., Pillaca, M., Delgado, S., Lee, D., Rahman, N., Fernandez, L. E., Bernhardt, E. S., and Kiefer, A. M.: Tracing atmospheric mercury from artisanal and small-scale gold mining, Environ. Sci. Technol., 59, 5021–5033, https://doi.org/10.1021/acs.est.4c10521, 2025.
Tang, B., Chen, J., Wang, Z., Qin, P., and Zhang, X.: Mercury accumulation response of rice plant (Oryza sativa L.) to elevated atmospheric mercury and carbon dioxide, Ecotoxicol. Environ. Saf., 224, 112628, https://doi.org/10.1016/j.ecoenv.2021.112628, 2021a.
Tang, W., Bai, X., Zhou, Y., Sonne, C., Wu, M., Lam, S. S., Hintelmann, H., Mitchell, C. P. J., Johs, A., Gu, B., Nunes, L., Liu, C., Feng, N., Yang, S., Rinklebe, J., Lin, Y., Chen, L., Zhang, Y., Yang, Y., and Zhong, H.: A hidden demethylation pathway removes mercury from rice plants and mitigates mercury flux to food chains, Nat. Food, 5, 72–82, https://doi.org/10.1038/s43016-023-00910-x, 2024.
Tang, W., Wu, M., Li, P., and Zhong, H.: Demethylation by reactive oxygen species lowers methylmercury accumulation in rice, J. Agric. Food Chem., 73, 8775–8783, https://doi.org/10.1021/acs.jafc.5c01324, 2025.
Tang, Z., Fan, F., Deng, S., and Wang, D.: Mercury in rice paddy fields and how some agricultural activities affect the translocation and transformation of mercury – A critical review, Ecotoxicol. Environ. Saf., 202, 110950, https://doi.org/10.1016/j.ecoenv.2020.110950, 2020.
Tang, Z., Fan, F., Wang, X., Shi, X., and Wang, D.: Understanding the effects of long-term different fertilizer applications on methylmercury accumulation in rice (Oryza sativa L.) plants, Sci. Total Environ., 777, 146125, https://doi.org/10.1016/j.scitotenv.2021.146125, 2021b.
Taux, K., Kraus, T., and Kaifie, A.: Mercury exposure and its health effects in Workers in the Artisanal and Small-Scale Gold Mining (ASGM) Sector – A Systematic Review, Int. J. Environ. Res. Public Health, 19, 2081, https://doi.org/10.3390/ijerph19042081, 2022.
Taylor, H., Appleton, J. D., Lister, R., Smith, B., Chitamweba, D., Mkumbo, O., Machiwa, J. F., Tesha, A. L., and Beinhoff, C.: Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining centre, Geita District, Tanzania, Sci. Total Environ., 343, 111–133, https://doi.org/10.1016/j.scitotenv.2004.09.042, 2005.
Telmer, K. H. and Veiga, M. M.: World emissions of mercury from artisanal and small scale gold mining, in: Mercury Fate and Transport in the Global Atmosphere, edited by: Mason, R. and Pirrone, N., Springer, Boston (USA), 131–172, https://doi.org/10.1007/978-0-387-93958-2_6, 2009.
Teschner, B. A.: “Orpaillage pays for everything”: How artisanal mining supported rural institutions following Mali's coup d'état, Futures, 62, 140–150, https://doi.org/10.1016/j.futures.2014.04.016, 2014.
Thomas, M. J., Veiga, M. M., Marshall, B., and Dunbar, W. S.: Artisanal gold supply chain: Measures from the Ecuadorian Government, Resour. Policy, 64, 101505, https://doi.org/10.1016/j.resourpol.2019.101505, 2019.
Timsina, S., Hardy, N. G., Woodbury, D. J., Ashton, M. S., Cook-Patton, S. C., Pasternack, R., and Martin, M. P.: Tropical surface gold mining: A review of ecological impacts and restoration strategies, Land Degrad. Dev., 33, 3661–3674, https://doi.org/10.1002/ldr.4430, 2022.
Tomiyasu, T., Hamada, Y. K., Baransano, C., Kodamatani, H., Matsuyama, A., Imura, R., Hidayati, N., and Rahajoe, J. S.: Mercury concentrations in paddy field soil and freshwater snails around a small-scale gold mining area, West Java, Indonesia, Toxicol. Environ. Health Sci., 12, 23–29, https://doi.org/10.1007/s13530-020-00045-7, 2020.
Tschakert, P.: Recognizing and nurturing artisanal mining as a viable livelihood, Resour. Policy, 34, 24–31, https://doi.org/10.1016/j.resourpol.2008.05.007, 2009.
Tsui, M. T.-K., Blum, J. D., and Kwon, S. Y.: Review of stable mercury isotopes in ecology and biogeochemistry, Sci. Total Environ., 716, 135386, https://doi.org/10.1016/j.scitotenv.2019.135386, 2020.
Ullrich, S. M., Tanton, T. W., and Abdrashitova, S. A.: Mercury in the aquatic environment: A review of factors affecting methylation, Crit. Rev. Environ. Sci. Technol., 31, 241–293, https://doi.org/10.1080/20016491089226, 2001.
UNEP: Global Mercury Assessment 2013: Sources, emissions, releases, and environmental transport, United Nations Environmental Programme, Kenya (NGA), https://www.unep.org/resources/report/global-mercury-assessment-2013-sources-emissions-releases-and-environmental (last access: 24 June 2024), 2013.
UNEP: Estimating Mercury Use and Documenting Practices in Artisanal and Small-scale Gold Mining (ASGM) – Methods and Tools Version 1.0, United Nations Environmental Programme, Kenya (NGA), https://wedocs.unep.org/20.500.11822/22894 (last access: 24 June 2024), 2017.
USEPA: Risk Assessment Guidance for Superfund. Volume 1: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment), Office of Superfund Remediation and Technology Innovation, USEPA: Washington DC (USA), EPA/540/R/99/005, 1–156, https://www.epa.gov/sites/production/files/2015-09/documents/part_e_final_revision_10-03-07.pdf (last access: 10 July 2024), 2004.
Veiga, M. and Baker, R.: Global mercury project. Protocols for environmental and health assessment of mercury released by artisanal and small-scale gold miners, United Nations Industrial Development Organization (UNIDO) Publishing, Vienna (AUT), https://iwlearn.net/documents/4897 (last access: 24 June 2024), 2004.
Veiga, M. M. and Gunson, A. J.: Gravity concentration in artisanal gold mining, Minerals, 10, 1026, https://doi.org/10.3390/min10111026, 2020.
Veiga, M. M. and Hinton, J.: Abandoned artisanal gold mines in the Brazilian Amazon: A legacy of mercury pollution, Nat. Resour. Forum, 26, 15–26, https://doi.org/10.1111/1477-8947.00003, 2002.
Veiga, M. M. and Marshall, B.: The Colombian artisanal mining sector: Formalization is a heavy burden, Extr. Ind. Soc., 6, 223–228, https://doi.org/10.1016/j.exis.2018.11.001, 2019.
Veiga, M. M., Meech, J. A., and Hypolito, R.: Educational measures to address mercury pollution from gold-mining activities in the Amazon, Ambio, 24, 216–220, 1995.
Veiga, M. M., Maxson, P. A., and Hylander, L. D.: Origin and consumption of mercury in small-scale gold mining, J. Clean. Prod., 14, 436–447, https://doi.org/10.1016/j.jclepro.2004.08.010, 2006.
Veiga, M. M., Angeloci-Santos, G., and Meech, J.: Review of barriers to reduce mercury use in artisanal gold mining, Extr. Ind. Soc., 1, 351–361, https://doi.org/10.1016/j.exis.2014.03.004, 2014.
Verbrugge, B. and Geenen, S. (Eds.): Global gold production touching ground: Expansion, informalization, and technological innovation, Springer Nature, Cham, CHE, 379, https://doi.org/10.1007/978-3-030-38486-9, 2020.
Vieira, R.: Mercury-free gold mining technologies: possibilities for adoption in the Guianas, J. Clean. Prod., 14, 448–454, https://doi.org/10.1016/j.jclepro.2004.09.007, 2006.
Vreman, K., Van Der Veen, N. G., Van der Molen, E. J., and De Ruig, W. G.: Transfer of cadmium, lead, mercury and arsenic from feed into milk and various tissues of dairy cows: chemical and pathological data, Neth. J. Agric. Sci., 34, 129–144, https://doi.org/10.18174/njas.v34i2.16799, 1986.
Wagner, L. and Hunter, M.: Links between artisanal and small-scale gold mining and organized crime in Latin America and Africa, in: Illegal Mining: Organized Crime, Corruption, and Ecocide in a Resource-Scarce World, edited by: Zabyelina, Y. and van Uhm, D., Springer Nature, Berlin (GER), 77–104, https://doi.org/10.1007/978-3-030-46327-4_4, 2020.
Wang, J., Shaheen, S. M., Jing, M., Anderson, C. W. N., Swertz, A.-C., Wang, S.-L., Feng, X., and Rinklebe, J.: Mobilization, methylation, and demethylation of mercury in a paddy soil under systematic redox changes, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.0c07321, 2021a.
Wang, K., Liu, G., and Cai, Y.: Possible pathways for mercury methylation in oxic marine waters, Crit. Rev. Environ. Sci. Technol., 52, 3997–4015, https://doi.org/10.1080/10643389.2021.2008753, 2021b.
Wang, X., Li, B., Tam, N. F.-Y., Huang, L., Qi, X., Wang, H., Ye, Z., Meng, M., and Shi, J.: Radial oxygen loss has different effects on the accumulation of total mercury and methylmercury in rice, Plant Soil, https://doi.org/10.1007/s11104-014-2239-x, 2014.
Wang, X., Tam, N. F.-Y., He, H., and Ye, Z.: The role of root anatomy, organic acids and iron plaque on mercury accumulation in rice, Plant Soil, 394, 301–313, https://doi.org/10.1007/s11104-015-2537-y, 2015.
Wang, X., Bao, Z., Lin, C. J., Yuan, W., and Feng, X.: Assessment of global mercury deposition through litterfall, Environ. Sci. Technol., 50, 8548–8557, https://doi.org/10.1021/acs.est.5b06351, 2016.
Wang, X., Yuan, W., Lin, C. J., Luo, J., Wang, F., Feng, X., Fu, X., and Liu, C.: Underestimated sink of atmospheric mercury in a deglaciated forest chronosequence, Environ. Sci. Technol., 54, 8083–8093, https://doi.org/10.1021/acs.est.0c01667, 2020.
Wilson, M., Renne, E., Roncoli, C., Agyei-Baffour, P., and Tenkorang, E.: Integrated assessment of artisanal and small-scale gold mining in Ghana – part 3: Social Sciences and Economics, Int. J. Environ. Res. Public Health, 12, 8133–8156, https://doi.org/10.3390/ijerph120708133, 2015.
Windham-Myers, L., Marvin-DiPasquale, M., Krabbenhoft, D. P., Agee, J. L., Cox, M. H., Heredia-Middleton, P., Coates, C., and Kakouros, E.: Experimental removal of wetland emergent vegetation leads to decreased methylmercury production in surface sediment, J. Geophys. Res., 114, https://doi.org/10.1029/2008JG000815, 2009.
Windham-Myers, L., Marvin-DiPasquale, M., Stricker, C. A., Agee, J. L., Kieu, L. H., and Kakouros, E.: Mercury cycling in agricultural and managed wetlands of California, USA: Experimental evidence of vegetation-driven changes in sediment biogeochemistry and methylmercury production, Sci. Total Environ., https://doi.org/10.1016/j.scitotenv.2013.05.028, 2013.
Wohlgemuth, L., Osterwalder, S., Joseph, C., Kahmen, A., Hoch, G., Alewell, C., and Jiskra, M.: A bottom-up quantification of foliar mercury uptake fluxes across Europe, Biogeosciences, 17, 6441–6456, https://doi.org/10.5194/bg-17-6441-2020, 2020.
Wohlgemuth, L., Rautio, P., Ahrends, B., Russ, A., Vesterdal, L., Waldner, P., Timmermann, V., Eickenscheidt, N., Fürst, A., Greve, M., Roskams, P., Thimonier, A., Nicolas, M., Kowalska, A., Ingerslev, M., Merilä, P., Benham, S., Iacoban, C., Hoch, G., Alewell, C., and Jiskra, M.: Physiological and climate controls on foliar mercury uptake by European tree species, Biogeosciences, 19, 1335–1353, https://doi.org/10.5194/bg-19-1335-2022, 2022.
World Gold Council: Gold Spot Prices, https://www.gold.org/goldhub/data/gold-prices, last access: 8 October 2025.
Wu, Q., Hu, H., Meng, B., Wang, B., Poulain, A. J., Zhang, H., Liu, J., Bravo, A. G., Bishop, K., Bertilsson, S., and Feng, X.: Methanogenesis is an important process in controlling MeHg concentration in rice paddy soils affected by mining activities, Environ. Sci. Technol., 54, 13517–13526, https://doi.org/10.1021/acs.est.0c00268, 2020.
Xia, J., Wang, J., Zhang, L., Anderson, C., Wang, X., Zhang, H., Dai, Z., and Feng, X.: Screening of native low mercury accumulation crops in a mercury-polluted mining region: Agricultural planning to manage mercury risk in farming communities, J. Clean. Prod., 262, 121324, https://doi.org/10.1016/j.jclepro.2020.121324, 2020.
Xu, X., Zhao, J., Li, Y., Fan, Y., Zhu, N., Gao, Y., Li, B., Liu, H., and Li, Y.-F.: Demethylation of methylmercury in growing rice plants: An evidence of self-detoxification, Environ. Pollut., 210, 113–120, https://doi.org/10.1016/j.envpol.2015.12.013, 2016.
Xu, X., Wang, J., and Feng, X.: Mercury contamination in mercury mining area in China, in: Methylmercury accumulation in rice, edited by: Feng, X., Wang, J., and Rinkelbe, J., CRC Press, Boca Raton, FL, 25–41, https://doi.org/10.1201/9781003404941, 2024.
Yanai, R. D., Yang, Y., Wild, A. D., Smith, K. T., and Driscoll, C. T.: New approaches to understand mercury in trees: radial and longitudinal patterns of mercury in tree rings and genetic control of mercury in maple sap, Water Air Soil Poll., 231, 248, https://doi.org/10.1007/s11270-020-04601-2, 2020.
Yin, R., Feng, X., and Meng, B.: Stable mercury isotope variation in rice plants (Oryza sativa L.) from the Wanshan mercury mining district, SW China, Environ. Sci. Technol., 47, 2238–2245, https://doi.org/10.1021/es304302a, 2013.
Yoshimura, A., Koyo, S., and Veiga, M. M.: Estimation of mercury losses and gold production by Artisanal and Small-Scale Gold Mining (ASGM), J. Sustain. Metall., 7, 1045–1059, https://doi.org/10.1007/s40831-021-00394-8, 2021.
Yu, M., Wang, Y., and Umair, M.: Minor mining, major influence: Economic implications and policy challenges of artisanal gold mining, Resour. Policy, 91, 104886, https://doi.org/10.1016/j.resourpol.2024.104886, 2024.
Yuan, W., Sommar, J., Lin, C. J., Wang, X., Li, K., Liu, Y., Zhang, H., Lu, Z., Wu, C., and Feng, X.: Stable isotope evidence shows re-emission of elemental mercury vapor occurring after reductive loss from foliage, Environ. Sci. Technol., 53, 651–660, https://doi.org/10.1021/acs.est.8b04865, 2018.
Zahir, F., Rizwi, S. J., Haq, S. K., and Khan, R. H.: Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol., 20, 351–360, https://doi.org/10.1016/j.etap.2005.03.007, 2005.
Zhao, H., Yan, H., Zhang, L., Sun, G., Li, P., and Feng, X.: Mercury contents in rice and potential health risks across China, Environ. Int., 126, 406–412, https://doi.org/10.1016/j.envint.2019.02.055, 2019.
Zhao, L., Qiu, G., Anderson, C. W. N., Meng, B., Wang, D., Shang, L., Yan, H., and Feng, X.: Mercury methylation in rice paddies and its possible controlling factors in the Hg mining area, Guizhou province, Southwest China, Environ. Pollut., 215, 1–9, https://doi.org/10.1016/j.envpol.2016.05.001, 2016.
Zhao, L., Meng, B., and Feng, X.: Mercury methylation in rice paddy and accumulation in rice plant: A review, Ecotoxicol. Environ. Saf., 195, 110462, https://doi.org/10.1016/j.ecoenv.2020.110462, 2020.
Zhou, J. and Obrist, D.: Global mercury assimilation by vegetation, Environ. Sci. Technol., 55, 14245–14257, https://doi.org/10.1021/acs.est.1c03530, 2021.
Zhou, J., Wang, Z., Zhang, X., and Gao, Y.: Mercury concentrations and pools in four adjacent coniferous and deciduous upland forests in Beijing, China, J. Geophys. Res. Biogeosci., 122, 1260–1274, https://doi.org/10.1002/2017JG003776, 2017.
Zhou, J., Obrist, D., Dastoor, A., Jiskra, M., and Ryjkov, A.: Vegetation uptake of mercury and impacts on global cycling, Nat. Rev. Earth Environ., 2, 269–284, https://doi.org/10.1038/s43017-021-00146-y, 2021.
Zhou, X. B. and Li, Y. Y.: Effect of iron plaque and selenium on mercury uptake and translocation in rice seedlings grown in solution culture, Environ. Sci. Pollut. Res., 26, 13795–13803, https://doi.org/10.1007/s11356-018-3066-z, 2019.
Zhu, H., Zhong, H., Evans, D., and Hintelmann, H.: Effects of rice residue incorporation on the speciation, potential bioavailability and risk of mercury in a contaminated paddy soil, J. Hazard. Mater., 293, 64–71, https://doi.org/10.1016/j.jhazmat.2015.03.051, 2015.
We use the notation IHg(II) throughout to differentiate inorganic and organic divalent Hg (MeHg). We choose this approach over the use of IHg, as “IHg” also includes Hg(0), which has distinct physicochemical properties and behaviour from all other Hg species.
Note the estimate of primary releases of Hg to aquatic systems does not include releases from ASGM activities as the lack of information and knowledge regarding these releases is, as yet, too large to produce a reliable estimate.
Epsilon values (i.e., ε202Hg) are indicative of the degree of fractionation between two samples or sample matrices. For example, if δ202Hg values for sample A and sample B are 1.00 ‰ and −1.00 ‰, respectively, then the ε202Hg would be −2.00 ‰ from A to B.
Reporting/method issues could also explain the very high crop/very low soil Hg concentration anomaly, but we could not identify any issues from the data provided.
Several other crops were studied, but each had data of only one sample and were not considered further.
There appears to be inconsistent use of parts-per notation (ppb/ppm). Contact author did not respond to inquiries about the potential data reporting issues.
Reporting/method issues could also explain the very high rice/very low soil Hg concentration anomaly, but we could not identify any issues from the data provided (the same anomaly was noted for other crops in this study; footnote iv).




