the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Wet and dry seasons modulate coastal coccolithophore dynamics off South-western Nigeria (Gulf of Guinea)
Falilu O. Adekunbi
Gerald Langer
Lucian O. Chukwu
Marta Alvarez
Shakirudeen Odunuga
Kai G. Schulz
Patrizia Ziveri
Coccolithophores are calcifying unicellular phytoplankton at the base of the marine food web, playing a key role in pelagic calcium carbonate production. While their sensitivity to environmental change is well established, their ecological importance in tropical coastal systems remains underexplored, particularly along the African coastline. Here, we present the first multi-seasonal assessment of living coccolithophore communities off Lagos, southwest Nigeria, in the Gulf of Guinea. Periodic sampling was conducted at three coastal stations from December 2018 to April 2021 to evaluate species composition, standing stocks, diversity, and ecological drivers. Coccolithophore abundances showed clear seasonal patterns, with significantly higher (p<0.05) standing stocks and diversity during the wet season. Total abundances ranged from 0.3×103 cells L−1 in the dry season to 5.5×103 cells L−1 in the wet season, with Gephyrocapsa oceanica dominating dry periods and Emiliania huxleyi prevailing during the wet season. Seasonal changes were linked to the migration of the Intertropical Convergence Zone (ITCZ), which modulates precipitation and current direction along the Gulf of Guinea. Interestingly, chlorophyll a concentrations appeared decoupled from coccolithophore abundance, suggesting other phytoplankton groups may drive primary productivity in this region. Despite regional differences in oceanographic settings, the observed standing stocks fall within the global range of coastal coccolithophore assemblages, supporting the hypothesis that these communities are shaped by a set of common ecological constraints. As tropical coastal regions already face multiple pressures from climate change, projected southward shifts of the ITCZ could alter precipitation regimes and current dynamics, with potential implications for coccolithophore community composition and coastal biogeochemical cycling.
- Article
(6258 KB) - Full-text XML
-
Supplement
(1369 KB) - BibTeX
- EndNote
Coccolithophores are a dominant group of calcareous phytoplankton. They play an important role in global biogeochemical cycling and account for up to 90 % of pelagic calcium carbonate production in the open ocean (Ziveri et al., 2023, 2025). They are a key component of the marine food web, and their significance in open ocean areas has long been recognised (Balch et al., 2019; Li et al., 2024; Poulton et al., 2017). More recently their ecological role in coastal areas has been emphasized (e.g., Addante et al., 2023; Godrijan et al., 2018), as coastal waters often display considerable seasonal and interannual variability. This is due to the interplay of land-sea physical and biogeochemical processes such as water mass mixing, coastal geomorphology, river inputs and runoff, as well as variability in primary productivity and respiration (Carstensen and Duarte, 2019). These coastal environmental parameters, that mainly drive coccolithophore seasonality, vary with geographic and oceanographic settings (Godrijan et al., 2018; Keuter et al., 2023; Priyadarshani et al., 2019). Sometimes dominating effects can be identified, e.g. seasonal upwelling (Ausín et al., 2018; Fiúza, 1984; Moita et al., 2003; Silva et al., 2008), or seasonal mixing (Keuter et al., 2022; Šupraha et al., 2016). However, despite the crucial role coccolithophores play as marine primary producers, a limited number of studies have addressed their region-specific seasonal variability in neritic environments (Bonomo et al., 2014, 2018a, b; O'Brien et al., 2013). This is especially true for African coastal areas, which are often overlooked in coccolithophores research. In particular, no study to date has investigated coccolithophores in the Gulf of Guinea, along the western coast of central Africa, where previous research has instead focused on diatoms and dinoflagellates as the dominant primary producers (Anang, 1979; Issifou et al., 2014; Koffi et al., 2024; Seu-Anoï et al., 2011).
Climate change and anthropogenic activities are profoundly altering coastal ecosystems (He and Silliman, 2019), especially in tropical regions (Kleypas, 2019), where hydrographic conditions are closely linked to atmospheric circulation patterns (Li et al., 2016). In the Gulf of Guinea, climate is strongly influenced by the Intertropical Convergence Zone (ITCZ), a region where the Northeast and Southeast trade winds converge. The region exhibits pronounced seasonality, primarily driven by ITCZ shifts caused by differential heating of the land and ocean, which strongly influence rainfall patterns (Kang, 2020; Nwankwo, 1996; Odekunle and Eludoyin, 2008). This mechanism controls the location of the subtropical Atlantic biogeochemical divide, with a high-phosphate, low-iron system in the south, and a low-phosphate, high-iron system in the north (Schlosser et al., 2014). Recent projections suggest that the ITCZ is expected to narrow and shift southwards due to ongoing climate change (Mamalakis et al., 2021) while local observations have demonstrated a shift in rainfall pattern, intensity and frequency (Fasona et al., 2019), with potentially significant implications for tropical climate regimes and marine productivity. In the nearby Cabo Verde region, northwest of the Gulf of Guinea, previous studies have shown that the seasonal migration of the ITCZ is a key driver of coccolithophore species distribution (Narciso et al., 2021; Silva et al., 2013). This underscores the urgent need to better understand the dynamics and response of the primary producers, which form the base of the food web, to such environmental changes. In this study, we present the first periodic monitoring of coccolithophore communities and associated environmental parameters in coastal waters off Lagos, Nigeria, from December 2018 to April 2021, encompassing both the wet and dry seasons, characteristic of the region. The objectives were (1) to assess seasonal variability in coccolithophore abundance and community structure, and (2) to identify the environmental factors driving these dynamics, with the overarching goal of advancing our understanding of the ecology of this key group of marine primary producers in the Gulf of Guinea.
2.1 Study area
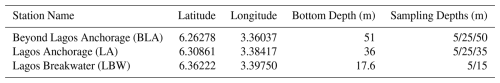
The study area is situated off the coast of Lagos (southwestern Nigeria) in the Gulf of Guinea (Fig. 1). The main oceanographic settings in the area are modulated by the equatorial currents of the Eastern Equatorial Atlantic Ocean: the equatorward Canary Current (CC) flowing along the western coasts of North Africa connects to the eastward North-Eastern Counter Current (NECC). The eastward Guinea Current (GC), which is an extension of the NECC (Hisard and Merle, 1980), flows along the coast of West Africa at a latitude of 4.5° N (Fig. 1). The coastal GC is dominant through the year, reaching a maximum velocity of 1 m s−1 from August to September in the vicinity of Cape Palmas in Côte d'Ivoire up to the West of Lagos (Da-Allada et al., 2021; Djakouré et al., 2017; Sohou et al., 2020). The GC transports warm (23–32 °C) and less salty water masses whose variability is driven by precipitation, wind stress and diffusion due to vertical shear (Alory et al., 2021; Awo et al., 2018; Binet, 1997). Off the Cameroun coast, the steep continental slope causes a reflection of the GC towards the west, forming the South Equatorial Current (SEC). Close to the SEC is a sub-surface eastward flow of the Equatorial Under Current (EUC) (van Bentum, 2012). During major upwelling in boreal summer (July to September) shoaling of the thermocline, brought about by the strengthening of the GC, drives a cooling of the surface ocean along the coast of Côte d'Ivoire and Ghana with a weak signal on the Western flank of Lagos coast (Oyewo et al., 1982; Sohou et al., 2020). This seasonal coastal cooling influences regional climate and also transports carbon-rich deep waters that seasonally drive variability in carbonate chemistry (Koffi et al., 2016). Primary production, also fuelled by upwelling, supports fisheries in the Gulf of Guinea (Djakouré et al., 2014; Zeller et al., 2020).
The rainfall in the Lagos coastal environment is characterized by a double maximum pattern where according to Nwankwo (1996) the dry season commences in November and extends until April while the wet season is from May to October with a break in August (Fig. 1). However, climate change has resulted already in local shifts in rainfall pattern, intensity and frequency, consequently the wet season now extends to November reaching a peak in July and September (Fasona et al., 2019). To take into account this shift, in this study the dry season is defined from December to April, while the wet season is defined from May to November. Seasonality in rainfall in this rainforest zone is brought about by continental scale movement of the ITCZ due to shifts in tropical maritime and continental air masses (Odekunle and Eludoyin, 2008) as well as seasonal migration of the southeast trade wind system (Nwankwo and Onyema, 2003).
2.2 Sampling strategy
Discrete samples of seawater were collected at three stations off Lagos in the Gulf of Guinea, onboard the M/V Sea Marshal of the Nigerian Maritime Administration and Safety Agency (NIMASA) (Fig. 1, Table 1). The three stations, Beyond Lagos Anchorage (BLA), Lagos Anchorage (LA) and Lagos Breakwaters (LBW) are respectively situated at 33.4, 22.2 and 11.1 km offshore, south of Lagos Harbour (Fig. 1). A total of 13 sampling campaigns were carried out between December 2018 and April 2021. A total of 99 samples were collected across the three stations at consistent depths ranging between 5 and 50 m (Table 1). The water samples were collected with a 2.5 L Teflon coated Niskin bottle (Model 1010, General Oceanics, Miami, FL, USA) with a graduated cable fastened to a 3 kg weight to allow sinking to target depth. A multiparametric YSI model Pro 1030 probe with an extended cable was used to measure the temperature (± 0.2 °C) and the salinity (± 0.1 PSU) at each target depth. Discrete seawater samples at target depths were taken to assess the coccolithophore community and target environmental variables (dissolved inorganic nutrients, chlorophyll a concentrations and seawater carbonate chemistry).
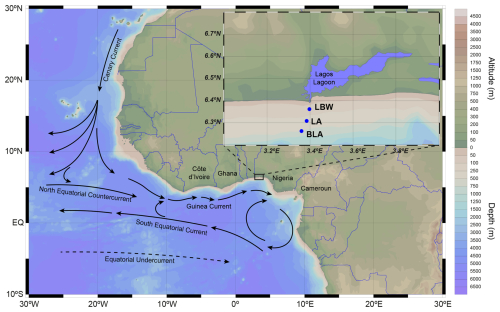
Figure 1General and local study area maps. The main surface currents in the Gulf of Guinea are presented in the general map (modified from Diop et al., 2014), while the 3 study sites are presented on the zoomed map in the upper right corner: Lagos Break-water (LBW), Lagos Anchorage (LA) and Beyond Lagos Anchorage (BLA). Map produced with Ocean Data View (Schlitzer, 2023).
2.3 Coccolithophore community
The seawater samples for coccolithophore community composition were stored shortly (< 5 h) in precleaned containers before filtering them in the laboratory. For each sample, an average of 2 L of seawater was filtered on a polycarbonate membrane (0.4 µm pore size, 47 mm diameter) for Scanning Electron Microscope (SEM) analyses, and cellulose acetate membrane (0.45 µm pore size, 47 mm diameter) for polarized light microscopy. The samples were then oven-dried at 60 °C for one hour before storage in plastic Petri dishes.
Coccolithophore quantification for standing stock and taxonomy was performed on an 8 mm × 8 mm piece of filter mounted on glass slides with Canada Balsam. The counting was performed with a ×1000 magnification Olympus polarized light microscope (model BX-51 U-FMP). A minimum of 100 (up to 513) coccospheres were counted per sample. The taxonomic identification was carried out following the coccolithophore light microscopy guide by Frada et al. (2010), the Nannotax3 website (http://ina.tmsoc.org/Nannotax3/, last access: 8 October 2025) and further confirmed with SEM. Based on coccosphere counts, absolute density in cells L−1 was calculated following the equation (Oviedo et al., 2014; Ziveri et al., 1995):
where: CD = Coccospheres concentration (cell L−1), A = Effective filtered area (mm2), N = Number of coccospheres counted, a = Analyzed area (mm2), V = volume of water filtered (L).
For SEM, a random section of the filter was stuck on stubs and coated (EMITECH K550X) with 95 % Gold and 5 % Palladium at a current of 20 mA for 3 min. Taxonomic identifications were performed with a MERLIN SEM at a magnification of ×3000, following the identification guide by Young et al. (2003).
2.4 Carbonate chemistry variables
Seawater samples for carbonate chemistry analysis were collected in acid-washed borosilicate bottles and preserved with HgCl2 following Dickson et al. (2007). Analyses were conducted at the INOCEN laboratory (IEO-CSIC, Spain) within six months of collection. For 2018–2020 samples, TA and DIC were measured; while for 2021, TA and pH were analysed due to equipment issues. DIC was measured coulometrically with a VINDTA 3D system, calibrated using certified reference material (CRM; batch #177), with an accuracy of ± 2.0 µmol kg−1 . TA was determined via potentiometric titration using a Titrando 909 system and CRM (batch #190), achieving ± 2 µmol kg−1 precision. pH was measured spectrophotometrically with m-cresol purple and a SHIMADZU UV-2600, at 25 ± 0.2 °C, yielding ± 0.002 pH unit accuracy. All pH values are reported on the total scale for in situ conditions. See Supplement for more details.
2.5 Dissolved inorganic nutrients
Dissolved inorganic nutrients samples were subsampled from the polyethylene bottles collected for the coccolithophore community sampling and an aliquot was stored at 4 °C until analyses in less than 24 h. The samples were filtered through a 0.45 µm Glass Fibre filter and the filtrate was used for the determination of NO, NO, PO, DIN (NO3-N, NO2-N, and NH3-N), and silicate (Si(OH)4) using a HACH DR 3900 spectrophotometer following (Rice et al., 2012).
2.6 Chlorophyll a
For each sample, 200 mL of seawater was filtered through a Whatman GF/F, glass fibre filter (nominal pore size 0.7 µm, 47 mm diameter). If immediate filtration was not possible, the water samples were kept on ice in the dark and filtered within 24 h. Filter homogenization and pigment extraction was carried out with 90 % acetone overnight in the dark at 4 °C, following standard protocols (Arar and Collins, 1997). The concentration of Chlorophyll a (Chl a) was then determined with a Lamotte Smart Spectro spectrophotometer. Results were validated with calibration standards and squared correlation coefficients r2>0.9990 were considered acceptable (Panagiotopoulos and Wurl, 2009).
2.7 External datasets integration
To put our results into perspective, existing dataset were used and processed. Monthly precipitation (mm h−1) between 20° N and 10° S over the study area, was retrieved from the Goddard Earth Sciences Data Information Services Center (GES DISC, Huffman et al., 2019). The data collected covers the study period with a resolution of 0.1° × 0.1°. The data of the monthly eastward seawater velocity were retrieved from the Copernicus Marine Environment Monitoring Service (CMEMS) through the Global Ocean Ensemble Physics Reanalysis (CMEMS, 2023). They cover the study period with a resolution of 0.25° × 0.25°. Both datasets were extracted with SeaDAS (version 8.4.0, https://seadas.gsfc.nasa.gov/, last access: 10 October 2023) using the pixel extraction tool. For precipitation, the data for each pixel in the area of interest was extracted for each month, while for the eastward sea water velocity, the data at the location of each station was extracted within a window size of 5 × 5 pixels (i.e.: 1.25° × 1.25°). The data extracted were processed and mapped with Ocean Data View (Schlitzer, 2023).
Global datasets for coccolithophore standing stocks (de Vries et al., 2020; O'Brien, 2012) were retrieved and processed in order to compare with our results. Only samples collected in coastal areas (< 50 km from the coastline) and with a maximum seafloor depth of 100 m, to match the characteristics of our study sites (Table 1), were processed. The selected samples were then organized and averaged for major oceanic regions (see Supplement for more details).
2.8 Statistical analyses
To test whether environmental parameters and coccolithophore community composition differed between the dry and wet seasons, and to identify which environmental conditions may influence coccolithophore communities, statistical analyses were performed using RStudio (v4.3.1; R Studio Team, 2020). The Shapiro-Wilk test was used to assess the normality of each variable within each season (Table S2). When normality was observed for a given parameter or species in both seasons, Welch two-sample t-test was applied to compare means between seasons (Table S3). When normality was not met for at least one season, the non-parametric Wilcoxon rank-sum test was used instead (Table S4). Finally, a principal component analysis (PCA) and Pearson correlations (Table S5) were performed between the environmental parameters and the coccolithophore data to explore patterns and interactions. See Supplement for more details.
3.1 Coccolithophore distribution
Only heterococcolithophores were identified in all the samples (15 taxa, Table S1, Fig. S1), as no holococcolithophore could be observed. Total coccosphere abundances varied through the studied period from a minimum of 0.3 × 103 cells L−1 which coincided with the dry season to a maximum of 47.5 × 103 cells L−1 in the wet season. Wet season abundances were on average higher (17.3 × 103 ± 11.4 × 103 cells L−1) compared to dry season records (10.6 × 103 ± 9.2 × 103 cells L−1) (Fig. 2d and f). In general, the average abundances were higher at the midpoint station LA (14.4 × 103 ± 12.3 × 103 cells L−1), followed by the farthest station BLA (13.9 × 103 ± 10.0 × 103 cells L−1) and the coastal station LBW (11.3 × 103 ± 9.31 × 103 cells L−1) (Fig. 2b).
Among the 15 identified taxa, only 7 exceeded 1 % relative abundance (Table S5). The assemblages were dominated by Emiliania huxleyi and Gephyrocapsa oceanica, together accounting for 83.2 % of the community (Fig. 2a). Emiliania huxleyi dominated during the wet season, representing an average of 66.5 % of the assemblage with a mean concentration of 11.4 × 103 ± 9.0 × 103 cells L−1 (range: 1.9–42.6 × 103 cells L−1) (Fig. 2e and f). During the dry season, G. oceanica dominated, representing an average of 63.7 % of the assemblage with a mean concentration of 6.7 × 103 ± 7.5 × 103 cells L−1 (range: 0.1–38.0 × 103 cells L−1) (Fig. 2c and d). During the dry season, only 3 other species exceeded 1 % of the assemblages: Gephyrocapsa ericsonii (11.3 %, 3.0 × 103 ± 4.2 × 103 cells L−1), Calciopapus rigidus (1.7 %, 0.3 × 103 ± 0.3 × 103 cells L−1) and Syracosphaera tumularis (1.2 %, 0.26 × 103 ± 0.23 × 103 cells L−1) (Fig. 2c and d). During the wet season, 4 other species exceeded 1 % of the assemblage: S. tumularis (8.2 %, 2.93 × 103 ± 5.93 × 103 cells L−1), Umbellicosphaera hulburtiana (3.0 %, 0.67 × 103 ± 0.85 × 103 cells L−1), C. rigidus (2.8 %, 0.73 × 103 ± 0.64 × 103 cells L−1) and Discosphaera tubifera (1.2 %, 0.59 × 103 ± 1.10 × 103 cells L−1) (Fig. 2e and f).
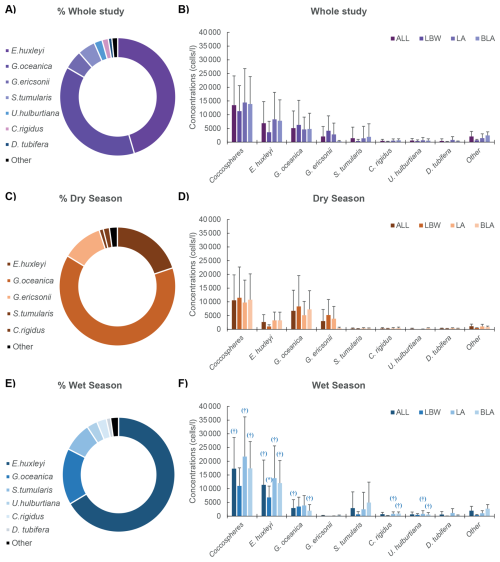
Figure 2Coccolithophore distribution during the dry and wet seasons: Left panels (A, C, E), from top to bottom, show the average relative abundances of the dominant species (contributing >1 % of the coccolithophore assemblage) for (A) the whole study period, (C) the dry season and (E) the wet season respectively. Right panels, from top to bottom, show the average absolute abundances of the dominant species for the 3 stations together (ALL) and each individual station (LBW, LA and BLA), for (B) the whole study period, (D) the dry season and (F) the wet season respectively. The error bars (1σ standard deviation) only show the upper bound for visibility. Significative difference (p-value <0.05) between the two seasons is given by the (*) and the (†) on the lower right panel, respectively for the Welch two-sample test and the Wilcoxon sum rank test (see Sect. 2.8 Statistical analysis for more details).
Spatially, E. huxleyi reached its peak abundance at the intermediate LA station during the wet season (Fig. 2f), while G. oceanica was most abundant at coastal station LBW during the dry season. For the minor species, G. ericsonii is the only one to show its highest abundances during the dry season at the coastal LBW station. The other minor species have all their highest abundances during the wet season: S. tumularis and C. rigidus at the offshore station BLA, and D. tubifera and U. hulburtiana at the intermediate station LA. This is reflected in a higher species richness during the wet season for each station (Table 2), as well as in coccolithophore diversity with higher Shannon diversity index (H′) during the wet (H′ = 1.62) compared to the dry season (H′ = 1.23) (Table 2, Fig. S2). The highest diversity for the wet season was recorded at the intermediate station LA (H′ = 1.76) and at the most coastal LBW station for the dry season (H′ = 1.28). Although the coccolithophore diversity in the coastal waters of the Gulf of Guinea are relatively low, it remains in the same range as other coastal sites (e.g. Balestra et al., 2008, H′ = 0.00–2.17; Dimiza et al., 2014, H′ = 0.14–1.42; Luan et al., 2016, H′ = 0.7–3.3).
3.2 Environmental variables
In general, the environmental variables measured (Table S6) along with the coccolithophore concentrations showed minimal seasonal variations with statistically significant differences mainly for salinity, silicate and precipitation (Fig. 3, Tables S3, S4). Slight differences, though not significant, were also observed: the temperature and the nitrate concentrations were the only variables that had slightly higher average values during the dry season, with 28.25 ± 1.70 °C and 32.34 ± 24.75 µmol kg−1 respectively, compared to the wet season, with 27.80 ± 2.49 °C and 26.19 ± 19.20 µmol kg−1 respectively (Fig. 3a and h). In contrast DIC, pH and nitrite concentrations showed an opposite trend with slightly higher values during the wet season, with 2013 ± 112, 7.94 ± 0.1 and 0.88 ± 1.80 µmol kg−1 respectively, compared to the dry season with 2004 ± 112, 7.93 ± 0.1 and 0.40 ± 0.73 µmol kg−1 respectively (Fig. 3b, d, f and i). The chlorophyll a and phosphate concentrations had on average very similar values between the dry and wet season (Fig. 3c and g), with respectively 11.34 ± 6.18 and 11.35 ± 5.72 µg L−1 for the former and 2.28 ± 3.78 and 2.25 ± 3.23 µmol kg−1 for the latter. There were differences between the 3 stations as most of the time, they showed spatial and temporal variations with no consistent pattern. As an example, temperature (Fig. 3a), pH (Fig. 3f), and chlorophyll a (Fig. 3c) were on average higher during the dry season at the coastal LBW and intermediate LA stations, while for the offshore BLA station, the waters are warmer during the wet season (Fig. 3a).
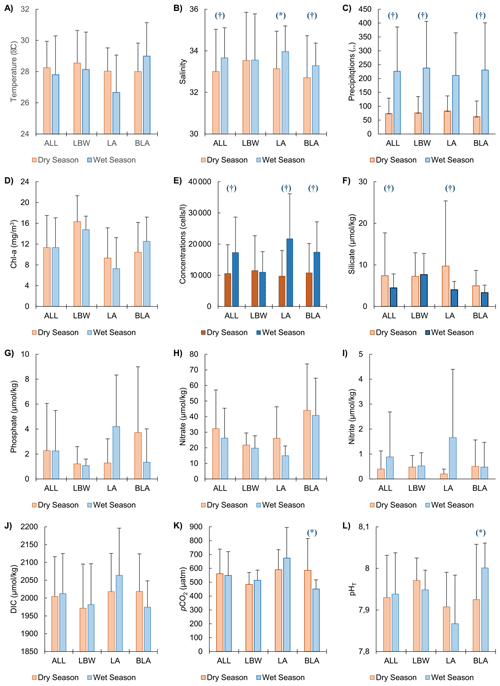
Figure 3Environmental parameter during the dry and wet seasons: Mean dry and wet season environmental parameters for the three sampled stations together (ALL) and each individual station (LBW, LA and BLA see Table 1). (A) Temperature, (B) Salinity, (C) Precipitations, (D) Chlorophyll a (Chl a), (E) Coccosphere concentrations, (F) Silicate, (G) Phosphate, (H) Nitrate and (I) Nitrite, (J) Dissolved inorganic carbon (DIC), (K) Partial pressure of carbon dioxide (pCO2) and (L) pH on the total scale (pHT). The central panel (coccosphere concentrations) have darker colours to facilitate the comparison with the environmental parameters. The error bars (1σ standard deviation) only show the upper bound for visibility. Significative difference (p-value <0.05) between the two seasons is given by the (*) and the (†), respectively for the Welch two-sample test and the Wilcoxon sum rank test (see Sect. 2.8 Statistical analysis for more details).
3.3 Statistical assessment
The Shapiro-Wilk normality testing (Table S2) indicated that only a few environmental parameters were normally distributed in both the dry and wet seasons, when all the stations were grouped: temperature, DIC, pHT, [HCO], [CO], Ωcalcite, Ωaragonite and chlorophyll a. Welch's two-sample t-tests conducted on these parameters (Table S3) revealed no statistically significant differences (p-value > 0.05) between the two seasons, though some significant differences could be observed for individual stations: salinity at LA station and, pHT, pCO2, Ωcalcite and Ωaragonite at station BLA. The Wilcoxon rank-sum test, applied to the remaining environmental variables and coccolithophore community data (Table S4), showed significant seasonal differences for only a few parameters, when all the stations were grouped: salinity, silicate, and precipitation. For the coccolithophore community, significant differences between the dry and wet seasons were found in total coccosphere abundance, the concentrations of E. huxleyi, G. oceanica, several minor species, and the diversity index and richness. Similar differences were observed for the stations LA and BLA while the coastal station LBW did not show any significant difference between the two seasons. Finally, the results of the Pearson correlations (Table S5) suggest that precipitation is the only environmental parameter showing significant correlation with the total coccolithophore abundances, and to a lesser degree with the abundances of E. huxleyi and G. oceanica. Interestingly, the highest correlation is observed between the abundances of G. oceanica and sea temperature during the wet season, which is not highlighted by the results of the PCA (Fig. 4).
Table 2Mean Shannon index (SI) and samples richness (Rich.) for the 3 stations and all together during the dry and wet season. For richness, the values into parenthesis represent the highest richness recorded. BLA: Beyond Lagos Anchorage, LA: Lagos Anchorage, LBW: Lagos Breakwater.
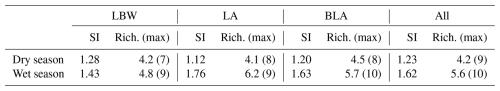
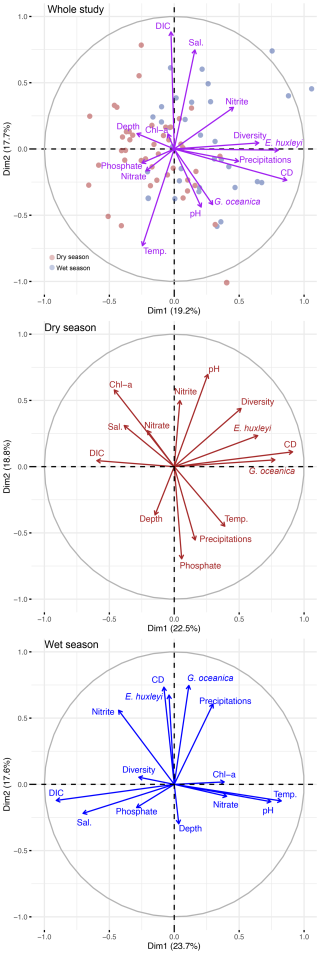
Figure 4Results of the principal component analysis (PCA) between the selected environmental variables, selected coccolithophore species and the diversity index, performed for the whole study (purple), the dry season (brown) and the wet season (blue). Sample distributions are shown for the whole study for both the dry (red) and wet (blue) seasons.
4.1 Factors modulating the coccolithophore community off the Nigerian coast
In the samples collected off the coasts of Lagos, the variations in total coccolithophore abundances (Fig. 2) and coccolithophore species richness and diversity (Table 2) show a clear seasonal pattern, with significantly higher abundance and diversity during the wet season (Table S4). The results of the PCA conducted on the whole dataset as well as for both the dry and wet seasons (Fig. 4) suggest a strong influence of precipitations on coccolithophore community structure. This is supported by the significant correlation between the total coccolithophore abundances (p-value < 0.01) as well as the abundances of E. huxleyi (p-value < 0.05) and G. oceanica (p-value < 0.05) with precipitation (Table S5). However, no clear relationship was observed between environmental parameters and total abundances, diversity or richness when the whole dataset was considered (Table S5). Especially, no correlation with temperature (Dimiza et al., 2015; Giraudeau et al., 1993; Hagino et al., 2000) or nutrients (Balestra et al., 2017; Schiebel et al., 2004), which are known to influence coccolithophore distribution and diversity, was found across the entire dataset. This is further supported by the limited seasonal changes in most environmental parameters at the sampling sites (Tables S3 and S4), with most of the environmental variables showing little to no seasonal variations (Fig. 3).
Although not detected by the PCA, a mere 0.5 °C seasonal temperature difference coincided with a marked shift in community composition, from E. huxleyi dominance in the wet season to G. oceanica dominance in the warmer dry season. The latter shows a significant correlation with temperature (Table S5). A significant negative correlation between assemblage diversity and richness and temperature is also observed during the wet season (Table S5). This ecological shift aligns with culture studies where temperature was identified as a potential driver of species composition by differently affecting growth rates (Gafar and Schulz, 2018). Notably, a global compilation of sediment coccolith abundances reported similar shifts around 27 °C, very close to the temperatures observed in our study (Gafar and Schulz, 2018).
Finally, the absence of holococcolithophores, the haploid phase of coccolithophores, can be explained by the fact that they are typically associated with stable, nutrient-poor open-ocean conditions. In contrast, the eutrophic and dynamic coastal waters off Lagos are not limiting for the heterococcolithophores, the diploid phase of coccolithophores, making holococcolithophores unlikely to occur in our samples (Guerreiro et al., 2023; Penales et al., 2025).
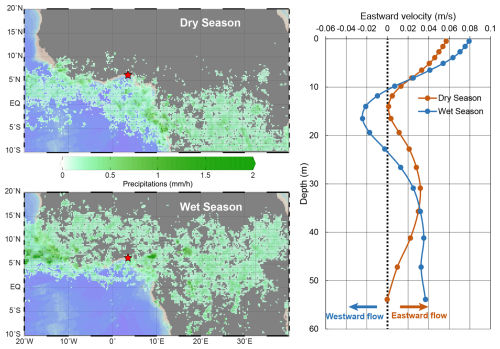
Figure 5ITCZ oscillation between the dry season (upper left) and the wet season (lower left) as depicted by the average precipitations (Huffman et al., 2019) for both seasons. Note that for visibility, average precipitations below 0.1 mm h−1 were not mapped, the red star refers to the sampling location. Maps produced with Ocean Data View (Schlitzer, 2023). On the right panel is shown the average eastward velocities (CMEMS, 2023) of the water masses at the sampling location for the dry (brown) and wet (blue) seasons. Negative values show a westward flow while positive values show an eastward flow.
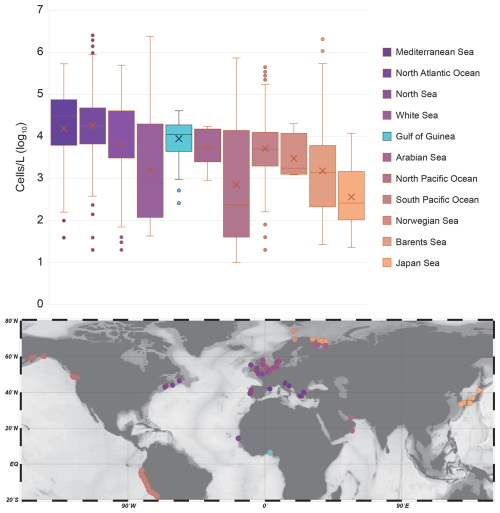
Figure 6Boxplots showing the distribution of cell concentrations (cells L−1) for each main oceanographic basin. Each box represents the interquartile range (1st to 3rd quartile), with the horizontal line indicating the median and whiskers extending to the minimum and maximum values within 1.5× the interquartile range. Outliers are shown as dots. Crosses indicate the average of each series. The y-axis is on a logarithmic scale (log10) to account for the high variability and wide range of concentrations across series. On the map are reported the locations of the samples used for each oceanographic basin.
4.2 Phytoplankton dynamics off Nigerian coast
Primary production in the ocean is sustained by phytoplankton functional types (i.e., diatoms, dinoflagellates and coccolithophores; O'Brien et al., 2013). Therefore, identifying the dominant coccolithophore taxa is essential. Chlorophyll a concentrations, which in the open ocean typically reflect coccolithophore cell density (Balch et al., 2019), appear decoupled from coccolithophore abundances in this region (Fig. 4d–e; Table S5), suggesting that other phytoplankton groups must drive chlorophyll a levels. Previous studies on phytoplankton communities in the inner shelf off Lagos have mainly focussed on diatoms (Nwankwo and Onyema, 2003), cyanobacteria (Onyema and Akanmu, 2018; Onyema and Popoola, 2013) and dinoflagellates (Akanmu and Onyema, 2020; Elegbeleye and Onyema, 2020), the latter making up only a minor contribution to the phytoplankton community. These studies established a diatom dominated seascape. Although maximum phytoplankton abundance during the dry season (i.e., diatoms, Onyema and Popoola, 2013) have been reported in the coastal waters off Lagos, our observations revealed lower coccolithophore concentrations during this period (Fig. 3e). We speculate that this may be linked to the general ecological succession between diatoms and coccolithophores in response to nutrient dynamics in coastal systems (Cermeño et al., 2011; Jin et al., 2019, 2022; Ziveri et al., 1995). Diatoms are known to be highly dependent on dissolved silica availability (Brzezinski et al., 2011; Xu et al., 2025). The pattern observed is supported by the higher average seawater concentrations of silicate (Fig. 3f) during the dry season (7.42 ± 10.28 µmol L−1) compared to the wet season (4.48 ± 3.33 µmol L−1). This supports the hypothesis of a successional dynamics between these important phytoplankton functional groups. Although several coccolithophore species also require silicon, limiting silicate concentrations are an order of magnitude lower than the average silicate concentrations measured here (Langer et al., 2021). Therefore, it is likely that coccolithophores were not affected by silicate while diatoms were.
4.3 The role of the ITCZ
In our study site the coccolithophore standing stocks are mainly driven by the alternation of the wet and dry seasons (Table S5). As shown by the results of the Wilcoxon rank-sum tests (Table S4), the precipitation rate, nitrite concentration, the total abundances, diversity and richness of coccolithophore species have significant wet to dry season differences (p-value < 0.05). Precipitation pattern, indicative of the dry and wet seasons, was the only factor found to positively correlate with the total coccolithophore abundance during the entire study period (Table S5). The Gulf of Guinea is directly influenced by the oscillation of the ITCZ, which follows the position of the thermal equator (i.e., the latitude of the hottest air; Ali et al., 2011). During the wet season (over the study area), the ITCZ moves northward up to 15° N, while during the dry season (over the study area), it moves southward down to approximately 5° N (Fig. 5). In the area, the water masses general flow direction changes according to the position of the ITCZ, as revealed by the eastward seawater velocity (CMEMS, 2023) shown in Fig. 5: during the dry season, the surface water masses tend to flow eastward, while during the wet season, they tend to flow westward. This suggests that the different coccolithophore communities observed during the dry and the wet season could have different geographical origins. During the dry season, the assemblage, dominated by G. oceanica could be brought offshore of Lagos by the intense Guinea current, possibly from the nearby upwelling system off Côte d'Ivoire and Ghana which shows a minor activity during the onset of the dry season (Ayissi et al., 2024). During the wet season, the assemblage dominated by E. huxleyi could be brought to the sampling site, from the nearby Niger Delta region, by an intensification of the westward current, possibly the Equatorial Undercurrent.
While the relatively short time series (∼ 2.5 years) limits our ability to fully resolve ITCZ-driven interannual variability, our findings represent the first documentation of coccolithophore seasonality in this understudied region. These results are supported by robust statistical analyses and are consistent with the documented influence of the ITCZ on phytoplankton assemblages in nearby regions, such as the Cape Verde archipelago (Narciso et al., 2021; Silva et al., 2013) and the equatorial Atlantic (Guerreiro et al., 2019).
4.4 The Gulf of Guinea and global coastal assemblages
Previous studies on coastal coccolithophore assemblages highlight significant variability driven by seasonal cycles, nutrient availability, and local oceanographic processes, all of which influence coccolithophore abundance and distribution (Addante et al., 2023; Godrijan et al., 2018). Although standing stocks can differ greatly depending on the specific coastal setting, our data indicate that the maximum abundances observed off Lagos are comparable to those reported in other shallow (< 100 m) coastal regions (de Vries et al., 2020; O'Brien, 2012). In these environments, coccolithophores coexist and compete with other phytoplankton groups, which may limit their proliferation, leading to shifts in community composition and moderate cell division rates. Despite very different oceanographic and climatic settings represented in the global dataset we extracted (1780 samples, Fig. 6), coccolithophore standing stocks off Lagos (mean: 1.3 × 104 ± 1.0 × 104 cells L−1 ; max: 4.8 × 104 cells L−1 during the wet season) are of the same order of magnitude as those found in other coastal environments such as the South Pacific Ocean (2.1 × 104 ± 5.7 × 104 cells L−1), the Barents Sea (3.0 × 104 ± 16.5 × 104 cells L−1), or the Mediterranean Sea (5.5 × 104 ± 7.5 × 104 cells L−1) (Fig. 6; Table S8). These similarities suggest that coccolithophore production in coastal systems may be subject to a set of common ecological constraints, such as higher turbidity, shallower mixed layers, greater nutrient fluctuations or even local pollution, regardless of the regional context.
In our study, both species richness and diversity fall in the lower range of those reported in comparable coastal settings (e.g., de Vries et al., 2020) and are markedly lower than values typically observed in oligotrophic oceanic regions such as the central Mediterranean Sea (Ziveri et al., 2014). This low diversity may reflect environmental limitations typical of coastal tropical zones, including variable salinity, episodic nutrient inputs, and high competition with other phytoplankton groups such as diatoms and cyanobacteria. Additionally, seasonal hydrodynamics and changes in water mass origin, as discussed in Sect. 4.3, may further limit the establishment of a more diverse coccolithophore community.
This study provides the first multi-seasonal assessment of coccolithophore abundance, diversity, and community structure in the coastal waters off Lagos, within the Gulf of Guinea. Our results reveal that coccolithophore standing stocks in this understudied tropical coastal region are comparable in magnitude to those observed in disparate coastal systems globally, despite notable differences in climatic and oceanographic settings. Seasonal variability driven by the Intertropical Convergence Zone (ITCZ), reflected in changes in precipitation, nutrient concentrations, and surface currents, emerges as a key environmental driver of coccolithophore community composition. While the wet season is associated with higher coccolithophore abundances, particularly of E. huxleyi, the dry season supports distinct assemblages dominated by G. oceanica, likely influenced by shifts in water mass origin. Interestingly, chlorophyll a concentrations, which are often used as proxies for coccolithophore biomass in the open ocean, appear decoupled from coccolithophore abundance in this coastal setting, highlighting the influence of other phytoplankton groups and local ecological dynamics. Overall, our findings support the hypothesis that coccolithophore assemblages in tropical coastal systems are shaped by a shared set of ecological pressures that constrain both abundance and diversity. These constraints appear to operate independently of regional characteristics, suggesting common mechanisms linked to environmental variability, resource competition, and hydrodynamic conditions. Future research integrating long-term time series, finer taxonomic resolution, and broader geographic coverage will be critical to better understand the ecological functioning of coccolithophores in coastal tropical environments and their role in regional biogeochemical cycles.
All the original data used and presented in this manuscript are available in the Supplement tables. The R code used for the analysis is included in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/bg-22-7865-2025-supplement.
FOA participated in conceptualization, formal analyses, investigation, methodology and writing, reviewing and editing of the manuscript. MG participated in conceptualization, formal analyses, sample analyses, supervision, writing original draft, writing, review and editing manuscript. GL participated in conceptualization, methodology and assisted in writing, reviewing, and editing the original draft. LOC assisted in conceptualization, investigation, supervision, writing original draft and writing and editing manuscript. MA carried out sample analyses, writing original draft, review and editing manuscript. SO assisted in investigation, supervision, writing original draft, writing, reviewing and editing. KGS contributed to manuscript writing, reviewing and editing. PZ assisted in conceptualization, methodology, assisted in funding acquisition, investigation, sample analyses, supervision, writing original draft, writing, review and editing manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We appreciate the Nigerian Maritime Administration and Safety Agency (NIMASA) for the provision of MV Sea Marshall for field campaigns. The Nigerian Institute for Oceanography and Marine Research (NIOMR) is also thanked for providing access to laboratory for the preparation of seawater for coccolithophore samples.
This research has been supported by the financial support of the Spanish Ministry of Science, Innovation and Universities, BIOCAL Project (PID2020-113526RB-I00), the BONITOS Project (International program DFG-AEI 2023 – Grant PCI2025-163190, 541693727), the Marine and Environmental Biogeosciences Research Group (MERS) Generalitat de Catalunya (2021 SGR 00640). This work contributes to ICTA-UAB “María de Maeztu” Programme for Units of Excellence of the Spanish Ministry of Science, Innovation and Universities (CEX2024-001506-M funded by MICIU/AEI/10.13039/501100011033). Falilu O. Adekunbi acknowledges funding from the Ocean Foundation (TOF), the Pier2Peer GOA-ON Mentorship Program. Gerald Langer from the Spanish Ministry of Universities through a Maria Zambrano grant. The inorganic chemical oceanography analysis were done by INOCEN (IEO-CSIC) lab technicians Elisa Fernández Guallart (PTA2016-12441-I), Mónica Castaño, Javier Gonzales Dequidt and Ruben Acerbi Amigo (PEJ2018-003991-A).
This paper was edited by Koji Suzuki and reviewed by three anonymous referees.
Addante, M., Grelaud, M., Langer, G., Maiorano, P., Bonomo, S., Álvarez, M., Johnson, R., and Ziveri, P.: Local hydrodynamic in coastal system affects the coccolithophore community at a short spatial scale, Mar. Micropaleontol., 185, https://doi.org/10.1016/j.marmicro.2023.102309, 2023.
Akanmu, R. T. and Onyema, I. C.: Phytoplankton composition and dynamics off the coast of Lagos south-west, Nigeria, Reg. Stud. Mar. Sci., 37, 101356, https://doi.org/10.1016/j.rsma.2020.101356, 2020.
Ali, K. E., Kouadio, K. Y., Zahiri, E.-P., Aman, A., Assamoi, A. P., and Bourles, B.: Influence of the Gulf of Guinea Coastal and Equatorial Upwellings on the Precipitations along its Northern Coasts during the Boreal Summer Period, Asian J. Appl. Sci., 4, 271–285, https://doi.org/10.3923/ajaps.2011.271.285, 2011.
Alory, G., Da-Allada, C. Y., Djakouré, S., Dadou, I., Jouanno, J., and Loemba, D. P.: Coastal Upwelling Limitation by Onshore Geostrophic Flow in the Gulf of Guinea Around the Niger River Plume, Front. Mar. Sci., 7, https://doi.org/10.3389/fmars.2020.607216, 2021.
Anang, E. R.: The seasonal cycle of the phytoplankton in the coastal waters of Ghana, Hydrobiologia, 62, 33–45, https://doi.org/10.1007/BF00012560, 1979.
Arar, E. J. and Collins, G. B.: Method 445.0: In vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence, Cincinnati: United States Environmental Protec-tion Agency, Office of Research and Development, National Exposure Research Laboratory, 1997.
Ausín, B., Zúñiga, D., Flores, J. A., Cavaleiro, C., Froján, M., Villacieros-Robineau, N., Alonso-Pérez, F., Arbones, B., Santos, C., de la Granda, F., G. Castro, C., Abrantes, F., Eglinton, T. I., and Salgueiro, E.: Spatial and temporal variability in coccolithophore abundance and distribution in the NW Iberian coastal upwelling system, Biogeosciences, 15, 245–262, https://doi.org/10.5194/bg-15-245-2018, 2018.
Awo, F. M., Alory, G., Da-Allada, C. Y., Delcroix, T., Jouanno, J., Kestenare, E., and Baloïtcha, E.: Sea Surface Salinity Signature of the Tropical Atlantic Interannual Climatic Modes, J. Geophys. Res.-Ocean., 123, 7420–7437, https://doi.org/10.1029/2018JC013837, 2018.
Ayissi, F. F. B. K., Da-Allada, C. Y., Baloïtcha, E., Worou, L. O., and Tilmes, S.: Changes in coastal upwelling in the northern Gulf of Guinea under Stratospheric Aerosol Injection, Reg. Stud. Mar. Sci., 76, 103607, https://doi.org/10.1016/j.rsma.2024.103607, 2024.
Balch, W. M., Bowler, B. C., Drapeau, D. T., Lubelczyk, L. C., Lyczkowski, E., Mitchell, C., and Wyeth, A.: Coccolithophore distributions of the North and South Atlantic Ocean, Deep-Sea Res. Pt. I, 151, 103066, https://doi.org/10.1016/j.dsr.2019.06.012, 2019.
Balestra, B., Marino, M., Monechi, S., Marano, C., and Locaiono, F.: Coccolithophore commu-nities in the Gulf of Manfredonia (Southern Adriatic Sea): Data from water and surface sediments, Micropaleontology, 54, 377–396, https://doi.org/10.47894/mpal.54.5.01, 2008.
Balestra, B., Grunert, P., Ausin, B., Hodell, D., Flores, J.-A., Alvarez-Zarikian, C. A., Hernandez-Molina, F. J., Stow, D., Piller, W. E., and Paytan, A.: Coccolithophore and benthic foraminifera distribution patterns in the Gulf of Cadiz and Western Iberian Margin during Integrated Ocean Drilling Program (IODP) Expedition 339, J. Mar. Syst., 170, 50–67, https://doi.org/10.1016/j.jmarsys.2017.01.005, 2017.
Binet, D.: Climate and pelagic fisheries in the Canary and Guinea currents 1964–1993: The role of trade winds and the southern oscillation, Oceanol. Acta, 20, 177–190, 1997.
Bonomo, S., Cascella, A., Alberico, I., Ferraro, L., Giordano, L., Lirer, F., Vallefuoco, M., and Marsella, E.: Coccolithophores from near the Volturno estuary (central Tyrrhenian Sea), Mar. Micropaleontol., 111, 26–37, https://doi.org/10.1016/j.marmicro.2014.06.001, 2014.
Bonomo, S., Cascella, A., Alberico, I., Lirer, F., Vallefuoco, M., Marsella, E., and Ferraro, L.: Living and thanatocoenosis coccolithophore communities in a neritic area of the central Tyrrhenian Sea, Mar. Micropaleontol., 142, 67–91, https://doi.org/10.1016/j.marmicro.2018.06.003, 2018a.
Bonomo, Sergio, Placenti, F., Zgozi, S., Torri, M., Quinci, E. M., Cuttitta, A., Genovese, S., Mazzola, S., Aronica, S., Barra, M., El Turki, A., Hamza, M., Uheshi, O., Bara, M., Assughayer, M., and Bonanno, A.: Relationship between coccolithophores and the physical and chemical oceanography of eastern Libyan coastal waters, Hydrobiologia, 821, 215–234, https://doi.org/10.1007/s10750-017-3227-y, 2018b.
Brzezinski, M. A., Baines, S. B., Balch, W. M., Beucher, C. P., Chai, F., Dugdale, R. C., Krause, J. W., Landry, M. R., Marchi, A., Measures, C. I., Nelson, D. M., Parker, A. E., Poulton, A. J., Selph, K. E., Strutton, P. G., Taylor, A. G., and Twining, B. S.: Colimitation of diatoms by iron and silicic acid in the equatorial Pacific, Deep-Sea Res. Pt. II, 58, 493–511, https://doi.org/10.1016/j.dsr2.2010.08.005, 2011.
Carstensen, J. and Duarte, C. M.: Drivers of pH Variability in Coastal Ecosystems, Environ. Sci. Technol., 53, 4020–4029, https://doi.org/10.1021/acs.est.8b03655, 2019.
Cermeño, P., Lee, J., Wyman, K., Schofield, O., and Falkowski, P.: Competitive dynamics in two species of marine phytoplankton under non-equilibrium conditions, Mar. Ecol. Prog. Ser., 429, 19–28, https://doi.org/10.3354/meps09088, 2011.
CMEMS: Global Ocean Ensemble Physics reanalysis, E.U. Copernicus Marine Service Information (CMEMS), Marine Data Store (MDS), https://doi.org/10.48670/moi-00024, 2023.
Da-Allada, C. Y., Agada, J., Baloïtcha, E., Hounkonnou, M. N., Jouanno, J., and Alory, G.: Causes of the Northern Gulf of Guinea Cold Event in 2012, J. Geophys. Res.-Ocean., 126, https://doi.org/10.1029/2021JC017627, 2021.
de Vries, J. C., Monteiro, F., Andruleit, H., Böckel, B., Baumann, K.-H., Cerino, F., Charalampopoulou, A., Cepek, M., Cros, L., D'Amario, B., Daniels, C. J., Dimiza, M. D., Estrada, M., Eynaud, F., Giraudeau, J., Godrijan Jelena, Guerreiro, C. V., Guptha, M. V. S., Thierstein, H. R., Haidar, A. T., Karatsolis, B. T., Kinkel, H., Luan, Q., Malinverno, E., Patil, S. M., Mohan, R., Poulton, A. J., Saavedra-Pellitero, M., Schiebel, R., Smith, H. E. K., Šupraha, L., Takahashi, K., Okada, H., Triantaphyllou, M., and Silver, M. W.: Global SEM coccolithophore abundance compilation, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.922933, 2020.
Dickson, A. G., Sabine, C. L., and Christian, J. R.: Guide to Best Practices for Ocean CO2 Measurements, edited by: Dickson, A. G., Sabine, C. L., and Christian, J. R., Sydney: North Pacific Marine Science Organization Sidney, British Columbia, ISBN 1-897176-07-4, 2007.
Dimiza, M., Triantaphyllou, M., and Malinverno, E.: New evidence for the ecology of Helicosphaera carteri in polluted coastal environments (Elefsis Bay, Saronikos Gulf, Greece), J. Nannopl. Res., 34, 37–43, https://doi.org/10.58998/jnr2081, 2014.
Dimiza, M. D., Triantaphyllou, M. V, Malinvemo, E., Psarra, S., Karatsolis, B.-T., Mara, P., Lagaria, A., and Gogou, A.: The composition and distribution of living coccolithophores in the Aegean Sea (NE Mediterranean), Micropaleontology, 61, 521–540, https://doi.org/10.47894/mpal.61.6.09, 2015.
Diop, S., Fabres, J., Pravettoni, R., Barusseau, J.-P., Descamps, C., and Ducrotoy, J.-P.: The Western and Central Africa Land–Sea Interface: A Vulnerable, Threatened, and Important Coastal Zone Within a Changing Environment, in: The Land/Ocean Interactions in the Coastal Zone of West and Central Africa, edited by: Diop, S., Barusseau, J. P., and Descamps, C., Estuaries of the World, Springer, Cham., 1–8, https://doi.org/10.1007/978-3-319-06388-1_1, 2014.
Djakouré, S. Penven, P., Bourlès, B., Veitch, J., and Koné, V.: Coastally trapped eddies in the north of the Gulf of Guinea, J. Geophys. Res.-Ocean., 119, 6805–6819, https://doi.org/10.1002/2014JC010243, 2014.
Djakouré, S., Penven, P., Bourlès, B., Koné, V., and Veitch, J.: Respective Roles of the Guinea Cur-rent and Local Winds on the Coastal Upwelling in the Northern Gulf of Guinea, J. Phys. Oceanogr., 47, 1367–1387, https://doi.org/10.1175/JPO-D-16-0126.1, 2017.
Elegbeleye, O. W. and Onyema, I. C.: Phytoplankton diversity and Stoichiometric nutrient limitation in the Lagos Harbour and adjacent sea, Southwestern Nigeria, Ife Journal of Science, 21, 139, https://doi.org/10.4314/ijs.v21i3.12, 2020.
Fasona, M. J., Muyiolu, S. K., Soneye, A. S., Ogundipe, O. T., Otusanya, O. O., Adekanmbi, O. H., Ade-onipekun, P. A., and Onuminya, T.: Temporal Analysis of the present and future climate of the Lagos coastal environment, Unilag Journal of Medicine, Science and Technology, 7, 113–128, 2019.
Fiúza, A. F. G.: Hydrology and dynamics of the Portuguese coastal waters, PhD. thesis, Universidade de Lisboa, Portugal, 1984.
Frada, M., Young, J., Cachão, M., Lino, S., Martins, A., Narciso, Á., Probert, I., and de Vargas, C.: A guide to extant coccolithophores (Calcihaptophycidae, Haptophyta) using light microscopy, J. Nannoplankton Res., 31, 58–112, https://doi.org/10.58998/jnr2094, 2010.
Gafar, N. A. and Schulz, K. G.: A three-dimensional niche comparison of Emiliania huxleyi and Gephyrocapsa oceanica: reconciling observations with projections, Biogeosciences, 15, 3541–3560, https://doi.org/10.5194/bg-15-3541-2018, 2018.
Giraudeau, J., Monteiro, P. M. S., and Nikodemus, K.: Distribution and malformation of living cocco-lithophores in the northern Benguela upwelling system off Namibia, Mar. Micropaleontol., 22, 93–110, https://doi.org/10.1016/0377-8398(93)90005-I, 1993.
Godrijan, J., Young, J. R., Marić Pfannkuchen, D., Precali, R., and Pfannkuchen, M.: Coastal zones as important habitats of coccolithophores: A study of species diversity, succession, and life-cycle phases, Limnol. Oceanogr., 63, 1692–1710, https://doi.org/10.1002/lno.10801, 2018.
Guerreiro, C. V., Baumann, K.-H., Brummer, G.-J. A., Korte, L. F., Sá, C., and Stuut, J.-B. W.: Transatlantic gradients in calcifying phytoplankton (coccolithophore) fluxes, Prog. Oceanogr., 176, 102140, https://doi.org/10.1016/j.pocean.2019.102140, 2019.
Guerreiro, C. V., Ferreira, A., Cros, L., Stuut, J. B., Baker, A., Tracana, A., Pinto, C., Veloso, V., Rees, A. P., Cachão, M. A. P., Nunes, T., and Brotas, V.: Response of coccolithophore communities to oceanographic and atmospheric processes across the North and Equatorial Atlantic, Front. Mar. Sci., 10, 1119488, https://doi.org/10.3389/fmars.2023.1119488, 2023.
Hagino, K., Okada, H., and Matsuoka, H.: Spatial dynamics of coccolithophore assemblages in the Equatorial Western-Central Pacific Ocean, Mar. Micropaleontol., 39, 53–72, https://doi.org/10.1016/S0377-8398(00)00014-1, 2000.
He, Q. and Silliman, B. R.: Climate Change, Human Impacts, and Coastal Ecosystems in the Anthropocene, Curr. Biol., 29, R1021–R1035, https://doi.org/10.1016/j.cub.2019.08.042, 2019.
Hisard, P. and Merle, J.: Onset of summer surface cooling in the Gulf of Guinea during GATE, Oceanography and Surface Layer Meteorology in the B/C Scale, Global Atmospheric Research Program Atlantic Tropical Experiment (Gata), GATE-2, 325–341, https://doi.org/10.1016/B978-1-4832-8366-1.50035-2, 1980.
Huffman, G. J., Stocker, E. F. , Bolvin, D. T., Nelkin, E. J., and Tan, J.: GPM IMERG Final Precipitation L3 Half Hourly 0.1° × 0.1° V06, Goddard Earth Sciences Data and Information Services Center (GES DISC) [data set], https://doi.org/10.5067/GPM/IMERG/3B-HH/06, 2019.
Issifou, L., Atanle, K., Radji, R., Lawson, H. L., Adjonou, K., Edorh, M. T., Kokutse, A. D., Mensah, A. A., and Kokou, K.: Checklist of tropical algae of Togo in the Guinean Gulf of West-Africa, Sci. Res. Essays, 9, 932–958, https://doi.org/10.5897/SRE2014.6113, 2014.
Jin, X., Liu, C., Xu, J., and Guo, X.: Coccolithophore Abundance, Degree of Calcification, and Their Contribution to Particulate Inorganic Carbon in the South China Sea, J. Geophys. Res.-Biogeo., 127, e2021JG006657, https://doi.org/10.1029/2021JG006657, 2022.
Jin, X. B., Zhao, Y. L., Zhang, Y. W., Wen, K., Lin, S., Li, J. R., and Liu, Z. F.: Two Production Stages of Coccolithophores in Winter as Revealed by Sediment Traps in the Northern South China Sea, JGR Biogeosciences, 124, 2335–2350, https://doi.org/10.1029/2019JG005070, 2019.
Kang, S. M.: Extratropical Influence on the Tropical Rainfall Distribution, Curr. Clim. Change Rep., 6, 24–36, https://doi.org/10.1007/s40641-020-00154-y, 2020.
Keuter, S., Silverman, J., Krom, M. D., Sisma-Ventura, G., Yu, J., Tsemel, A., Ben-Ezra, T., Sher, D., Reich, T., Koplovitz, G., and Frada, M. J.: Seasonal patterns of coccolithophores in the ultra-oligotrophic South-East Levantine Basin, Eastern Mediterranean Sea, Mar. Micropaleontol., 175, https://doi.org/10.1016/j.marmicro.2022.102153, 2022.
Keuter, S., Koplovitz, G., Torfstein, A., and Frada, M. J.: Two-year seasonality (2017, 2018), export and long-term changes in coccolithophore communities in the subtropical ecosystem of the Gulf of Aqaba, Red Sea, Deep-Sea Res. PT. I, 191, 103919, https://doi.org/10.1016/j.dsr.2022.103919, 2023.
Kleypas, J. A.: Climate change and tropical marine ecosystems: A review with an emphasis on coral reefs, Cuad. Inv. UNED, 11, 24–35, 2019.
Koffi, K. U., Konan, E. S., Hassoun, A. E. R., and Kouadio, Y.: Relationship between the carbonate system and phytoplankton community in the Gulf of Guinea-Africa, Front. Mar. Sci., 11, https://doi.org/10.3389/fmars.2024.1286338, 2024.
Koffi, U., Kouadio, G., and Kouadio, Y. K.: Estimates and Variability of the Air-Sea CO2 Fluxes in the Gulf of Guinea during the 2005–2007 Period, Open J. Mar. Sci., 06, 11–22, https://doi.org/10.4236/ojms.2016.61002, 2016.
Langer, G., Taylor, A. R., Walker, C. E., Meyer, E. M., Ben Joseph, O., Gal, A., Harper, G. M., Probert, I., Brownlee, C., and Wheeler, G. L.: Role of silicon in the development of complex crystal shapes in coccolithophores, New Phytol., 231, 1845–1857, https://doi.org/10.1111/nph.17230, 2021.
Li, S., Zhu, J., Jin, X., Feng, Y., Jiao, N., and Zhang, W.: Multifaceted contribution of coccolithophores to ocean carbon export, Ocean-Land-Atmos. Res., 3, 0049, https://doi.org/10.34133/olar.0049, 2024.
Li, X., Xie, S.-P., Gille, S. T., and Yoo, C.: Atlantic-induced pan-tropical climate change over the past three decades, Nat. Clim. Chang., 6, 275–279, https://doi.org/10.1038/nclimate2840, 2016.
Luan, Q., Liu, S., Zhou, F., and Wang, J.: Living coccolithophore assemblages in the Yellow and East China Seas in response to physical processes during fall 2013, Mar. Micropaleontol., 123, 29–40, https://doi.org/10.1016/j.marmicro.2015.12.004, 2016.
Mamalakis, A., Randerson, J. T., Yu, J.-Y., Pritchard, M. S., Magnusdottir, G., Smyth, P., Levine, P. A., Yu, S., and Foufoula-Georgiou, E.: Zonally contrasting shifts of the tropical rain belt in response to climate change, Nat. Clim. Chang., 11, 143–151, https://doi.org/10.1038/s41558-020-00963-x, 2021.
Moita, M. T., Oliveira, P. B., Mendes, J. C., and Palma, A. S.: Distribution of chlorophyll a and Gymnodinium catenatum associated with coastal upwelling plumes off central Portugal, Acta Oecol., 24, S125–S132, https://doi.org/10.1016/S1146-609X(03)00011-0, 2003.
Narciso, Á., Javidpour, J., Chi, X., Cachão, M., and Kaufmann, M.: Characterization of the coccolitho-phore community off Cabo Verde archipelago, including the Senghor Seamount (Eastern North At-lantic), Estuar. Coast. Shelf Sci., 250, 107146, https://doi.org/10.1016/j.ecss.2020.107146, 2021.
Nwankwo, D. I.: Phytoplankton diversity and succession in Lagos Lagoon, Nigeria, Arch. Hydrobiol., 135, 529–542, https://doi.org/10.1127/archiv-hydrobiol/135/1996/529, 1996.
Nwankwo, D. I. and Onyema, I. C.: A checklist of planktonic algae off Lagos coast, J. Scientif. Res. Dev., 9, 75–85, 2003.
O'Brien, C. J.: Global distributions of coccolithophores abundance and biomass – Gridded data product (NetCDF) – Contribution to the MAREDAT World Ocean Atlas of Plankton Functional Types, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.785092, 2012.
O'Brien, C. J., Peloquin, J. A., Vogt, M., Heinle, M., Gruber, N., Ajani, P., Andruleit, H., Arístegui, J., Beaufort, L., Estrada, M., Karentz, D., Kopczyńska, E., Lee, R., Poulton, A. J., Pritchard, T., and Widdicombe, C: Global marine plankton functional type biomass distributions: coccolithophores, Earth Syst. Sci. Data, 5, 259–276, https://doi.org/10.5194/essd-5-259-2013, 2013.
Odekunle, T. O. and Eludoyin, A. O.: Sea surface temperature patterns in the Gulf of Guinea: their implications for the spatio-temporal variability of precipitation in West Africa, Int. J. Climatol., 28, 1507–1517, https://doi.org/10.1002/joc.1656, 2008.
Onyema, I. C. and Akanmu, R. T.: Environmental variables, algal pigments and phytoplankton in the Atlantic Ocean off the coast of Badagry, Lagos, J. Aquat. Sci., 32, 171, https://doi.org/10.4314/jas.v32i1A.19, 2018.
Onyema, I. C. and Popoola, R. T.: The physico-chemical characteristics, chlorophyll a levels and phytoplankton dynamics of the east mole area of the Lagos Harbour, Lagos, J. Asian Sci. Res., 3, 995–1010, 2013.
Oviedo, A. M., Langer, G., and Ziveri, P.: Effect of phosphorus limitation on coccolith morphology and element ratios in Mediterranean strains of the coccolithophore Emiliania huxleyi, J. Exp. Mar. Biol. Ecol., 459, 105–113, https://doi.org/10.1016/j.jembe.2014.04.021, 2014.
Oyewo, E. O., Ajao, E. A., and Orekoya, T.: Seasonal variation in surface temperature and salinity around Lagos harbour, Nigeria, Nigerian Institute for Oceanography & Marine Research, Lagos, Technical Paper no. 10, http://hdl.handle.net/1834/1270 (last access: 2 July 2025), 1982.
Panagiotopoulos, C. and Wurl, O.: Spectrophotometric and Chromatographic Analysis of Carbohydrates in Marine Samples, in: Practical Guidelines for the Analysis of Seawater, Olivier Wurl Eds., CRC Press, https://doi.org/10.1201/9781420073072, 2009.
Penales, P. J. F., Skampa, E., Dimiza, M. D., Parinos, C., Velaoras, D., Pavlidou, A., Oikonomou, V. A., and Triantaphyllou, M. V.: Coccolithophore Assemblage Dynamics and Emiliania huxleyi Morphological Patterns During Three Sampling Campaigns Between 2017 and 2019 in the South Aegean Sea (Greece, NE Mediterrane-an), Geosciences, 15, 268, https://doi.org/10.3390/geosciences15070268, 2025.
Poulton, A. J., Holligan, P. M., Charalampopoulou, A., and Adey, T. R.: Coccolithophore ecology in the tropical and subtropical Atlantic Ocean: New perspectives from the Atlantic meridional transect (AMT) programme, Prog. Oceanogr., 158, 150–170, https://doi.org/10.1016/j.pocean.2017.01.003, 2017.
Priyadarshani, W. N. C., Ran, L., Wiesner, M. G., Chen, J., Ling, Z., Yu, S., and Ye, Y.: Seasonal and interannual variability of coccolithophore flux in the northern South China Sea, Deep-Sea Res. Pt. I, 145, 13–30, https://doi.org/10.1016/j.dsr.2019.01.004, 2019.
R Studio Team: RStudio: Integrated Development for R. RStudio, PBC, https://www.rstudio.com/ (last access: 20 March 2023), 2020.
Rice, E. W., Baird, R. B., Eaton, A. D., Clesceri, L. S., and Bridgewater, L.: Standard Methods for the Examination of Water and Waste Water, in: Journal of the North American Benthological Society (Vol. 12, Issue 3), edited by: Rice, E. W., Baird, R. B., Eaton, A. D., Clesceri, L. S., and Bridgewater, L., Washington: DC American Public Health Association American Water Works Association Water Environment Federation, https://doi.org/10.2105/smww.2882 2012.
Schiebel, R., Zeltner, A., Treppke, U. F., Waniek, J. J., Bollmann, J., Rixen, T., and Hemleben, C.: Distribution of diatoms, coccolithophores and planktic foraminifers along a trophic gradient during SW monsoon in the Arabian Sea, Mar. Micropaleontol., 51, 345–371, https://doi.org/10.1016/j.marmicro.2004.02.001, 2004.
Schlitzer, R.: Ocean Data View, Verion 5.6.6, https://odv.awi.de (last access: 2 July 2025), 2023.
Schlosser, C., Klar, J. K., Wake, B. D., Snow, J. T., Honey, D. J., Woodward, E. M. S., Lohan, M. C., Achter-berg, E. P., and Moore, C. M.: Seasonal ITCZ migration dynamically controls the location of the (sub)tropical Atlantic biogeochemical divide, P. Natl. Acad. Sci. USA, 111, 1438–1442, https://doi.org/10.1073/pnas.1318670111, 2014.
Seu-Anoï, N. M., Ouattara, A., Koné, Y. J.-M., and Gourène, G.: Seasonal distribution of phytoplank-ton in the Aby lagoon system, Ivory Coast, West Africa, Afr. J. Aquat. Sci., 36, 321–330, https://doi.org/10.2989/16085914.2011.643561, 2011.
Silva, A., Palma, S., and Moita, M. T.: Coccolithophores in the upwelling waters of Portugal: Four years of weekly distribution in Lisbon bay, Cont. Shelf Res., 28, 2601–2613, https://doi.org/10.1016/j.csr.2008.07.009, 2008.
Silva, A., Brotas, V., Valente, A., Sá, C., Diniz, T., Patarra, R. F., Álvaro, N. V., and Neto, A. I.: Coccolithophore species as indicators of surface oceanographic conditions in the vicinity of Azores islands, Estuar. Coast. Shelf Sci., 118, 50–59, https://doi.org/10.1016/j.ecss.2012.12.010, 2013.
Sohou, Z., Koné, V., Da-Allada, Y. C., Djakouré, S., Bourlès, B., Racape, V., Degbe, G., and Adje, C.: Seasonal and inter-annual ONSET Sea Surface Temperature variability along the northern coast of the Gulf of Guinea, Reg. Stud. Mar. Sci., 35, 101129, https://doi.org/10.1016/j.rsma.2020.101129, 2020.
Šupraha, L., Ljubešić, Z., Mihanović, H., and Henderiks, J.: Coccolithophore life-cycle dynamics in a coastal Mediterranean ecosystem: seasonality and species-specific patterns, J. Plankton Res., 38, 1178–1193, https://doi.org/10.1093/plankt/fbw061, 2016.
van Bentum, K. M.: The Lagos coast – Investigation of the long-term morphological impact of the Eko Atlantic City project, NCK-Days 2012: Crossing Borders in Coastal Research: Jubilee Conference Proceedings, https://doi.org/10.3990/2.199, 2012.
Xu, H., Chen, F., Luo, M., Zhang, X., Pan, K., and Liu, H.: Silicon limitation affects diatom's resistance to copepod grazing, J. Oceanol. Limnol., 43, 1201–1212, https://doi.org/10.1007/s00343-024-4142-5, 2025.
Young, J. R., Geisen, M., Cros, L., Kleijne, A., Sprengel, C., Probert, I., and Østergaard, J.: A guide to extant coccolithophore taxonomy, J. Nannoplankton Res., Special Issue, 1–132, 2003.
Zeller, D., Hood, L., Palomares, M. L. D., Sumaila, U. R., Khalfallah, M., Belhabib, D., Woroniak, J., and Pauly, D.: Comparative fishery yields of African Large Marine Ecosystems, Env. Dev., 36, 100543, https://doi.org/10.1016/j.envdev.2020.100543, 2020.
Ziveri, P., Thunell, R. C., and Rio, D.: Seasonal changes in coccolithophore densities in the Southern California Bight during 1991–1992, Deep-Sea Res. Pt. I, 42, 1881–1903, https://doi.org/10.1016/0967-0637(95)00089-5, 1995.
Ziveri, P., Passaro, M., Incarbona, A., Milazzo, M., Rodolfo-Metalpa, R., and Hall-Spencer, J. M.: Decline in Coccolithophore Diversity and Impact on Coccolith Morphogenesis Along a Natural CO2 Gradient, Biol. Bull., 226, 282–290, https://doi.org/10.1086/BBLv226n3p282, 2014.
Ziveri, P., Gray, W. R., Anglada-Ortiz, G., Manno, C., Grelaud, M., Incarbona, A., Rae, J. W. B., Subhas, A. V., Pallacks, S., White, A., Adkins, J. F., and Berelson, W.: Pelagic calcium carbonate production and shallow dissolution in the North Pacific Ocean, Nat. Commun., 14, 805, https://doi.org/10.1038/s41467-023-36177-w, 2023.
Ziveri, P., Langer, G., Chaabane, S., de Vries, J., Gray, W. R., Keul, N., Hatton, I. A., Manno, C., Norris, R., Pallacks, S., Young, J. Y., Anglada-Ortiz, G., Bianco, S., de Garidel-Thoron, T., Grelaud, M., Lucas A., Probert, I., and Mortyn, G.: Calcifying plankton: From biomineralization to glogal change, Science, 390, https://doi.org/10.1126/science.adq8520, 2025.