the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Reviews and syntheses: Carbon vs. cation based MRV of Enhanced Rock Weathering and the issue of soil organic carbon
Mathilde Hagens
Jens S. Hammes
Noah Planavsky
Philip A. E. Pogge von Strandmann
Tom Reershemius
Christopher T. Reinhard
Phil Renforth
Tim J. Suhrhoff
Sara Vicca
Arthur Vienne
Dieter Wolf-Gladrow
We discuss the “monitoring, reporting and verification” (MRV) strategy of Enhanced Weathering (EW) based on carbon accounting and argue that in open systems such as arable land, this approach is ill-suited to close the balance of all carbon fluxes. We argue for total alkalinity (TA) as the central parameter for the carbon based MRV of EW. However, we also stress that tracking alkalinity fluxes using a systems-level approach is best done by focusing on charge balance maintenance through time. We start by explaining the concept and history of alkalinity conceptualization for the oceans. The same analytical method first proposed for the oceans – titration with a strong acid – is now commonly used for porewaters in agricultural soils. We explain why this is an accurate analysis for ocean water and why it is unsuitable to record TA for porewaters in agricultural soils. We then introduce an alternative MRV based on cation accounting and finally discuss the fate of cations released from the weathering of basalt, soil cation dynamics and close by suggesting open research questions.
- Article
(1541 KB) - Full-text XML
- BibTeX
- EndNote
Relying on future atmospheric carbon dioxide removal (CDR) to avoid dangerous climate change while continuing to burn fossil fuels has been termed the “overshoot myth” and is extremely dangerous (e.g. Schleussner et al., 2024). The average atmospheric CO2 concentration reached 424.6 parts per million in 2024, 3.5 parts per million above 2023, and 52 % above pre-industrial levels (Friedlingstein et al., 2025). Kotz et al. (2024) estimate that, due to global warming, the world economy is committed to an income reduction of 19 % in the next 26 years. This equals USD 38 trillion which is six times higher than the abatement costs of limiting global warming to no more than 2 °C. Immediate emissions reduction through realisation of current national pledges, and continued strengthening of these pledges, is the only realistic way to limit global warming to well below 2 °C above pre-industrial levels – the goal set by the 2015 Paris Agreement on climate change (Liu and Raftery, 2021; Ou et al., 2021; Linow et al., 2022; Meinshausen et al., 2022; Dafnomilis et al., 2024; Ripple et al., 2024). Nonetheless, a consensus view is that to meet this goal, billions of tons of CDR will have to accompany emissions reduction, given the prevalence of hard-to-abate residual emissions (Luderer et al., 2018; Buck et al., 2023; Ipcc, 2023). For the 2 °C target without overshoot, the remaining carbon budget is around 1200 Gt CO2 (Lamboll et al., 2023). It has been suggested that if we wish to return to a safe climate for universal human habitation after reaching “net zero”, including the global South, we need to further lower the atmospheric CO2 levels to below 350 ppm (Hansen et al., 2023). Hence, CDR will have to become a major industry for multiple generations to come, in the order of the current fossil fuel industry. It is estimated that we will need to remove roughly 7–9 billion tonnes of CO2 per year by 2050 to meet the Paris climate goals (Peplow, 2024). McKinsey research (https://www.mckinsey.com/capabilities/sustainability/our-insights/carbon-removals-how-to-scale-a-new-gigaton-industry, last access: 18 December 2025), approximates that the value of the voluntary carbon market could grow to more than USD 50 billion by 2030 and that the CDR industry could be worth up to USD 1.2 trillion annually by 2050. The annual burden towards 350 ppm depends on the adopted timescale.
Given the scale of the challenge, it is imperative to consider how long-term natural regulation of atmospheric CO2 concentration (Appendix A) may be harnessed to bring anthropogenic CDR to scale, both via the oceanic and the terrestrial ecosystem. Although increasing and maintaining the amount of carbon stored in the biosphere and in soils is essential to meeting climate goals, reservoirs such as these with a CO2 storage period of less than 1000 years are insufficient for neutralising long-term residual fossil CO2 emissions under net zero emissions scenarios (Brunner et al., 2024).
In the absence of anthropogenic forcing, and on timescales of a few thousand years, variations in atmospheric CO2 concentration1 are driven by shifts in ocean circulation, the pelagic carbon cycle and the ratio of dissolved inorganic carbon (DIC) to alkalinity2 delivered to the oceans via river discharge. The resulting CO2 is then partitioned between the atmosphere and ocean. Without major carbon perturbations, this quasi steady state is modulated annually by the seasonality of the net primary productivity on land.
The current carbon perturbation by anthropogenic emissions exceeds the natural regulation speed of the oceanic buffering system (Maier-Reimer and Hasselmann, 1987; Hooss et al., 2001) and lowers its capacity (Hagens and Middelburg, 2016). Currently, ∼25 % of our annual CO2 emissions are taken up by the ocean, primarily by dissolution affecting the oceanic carbonate chemistry and, to a much smaller extent, the ocean's “biological carbon pump” (e.g., Siegenthaler and Sarmiento, 1993; Friedlingstein et al., 2023, 2025). More than 90 % of the excess insolation energy (“global warming”) is taken up by the ocean (Venegas et al., 2023). While lessening the impact of climate change on terrestrial ecosystems, CO2 and heat uptake comes at a high cost for marine ecosystems. Ocean acidification and heat waves (Frölicher et al., 2018) are now threatening marine life, notably coral reefs that are a hotspot for oceanic biodiversity. The effects of these perturbations could be partially ameliorated through the addition of alkalinity to the ocean. One way of generating this alkalinity is via the addition of pulverised silicate rock on arable land (“Enhanced Weathering” or EW). Because it is estimated that 25 % of the hard-to-abate-emissions will come from the agricultural sector (Edelenbosch et al., 2024), it would be proactive to already implement and scale up EW with the support of farmers. This can be achieved by developing business models based on carbon credits and the agronomic benefits related to EW that have been demonstrated in some studies (Manning and Theodoro, 2018; Swoboda et al., 2022).
The addition of crushed silicate rock to (arable) soils, its subsequent dissolution, its reaction with atmospheric and soil CO2, and the transport of the resulting weathering products to the ocean, constitute a promising CDR approach (termed “enhanced (rock) weathering” E(R)W) (Schuiling, 1990; Schuiling and Krijgsman, 2006; Hartmann et al., 2013; Amann et al., 2020; Beerling et al., 2020). Initial emissions associated with the supply chain of crushed rock are small (ca. 6 %–23 % e.g. Renforth, 2012) in comparison to the overall carbon dioxide removal from the atmosphere that can be expected to result from weathering over time. Additionally, the infrastructure required for EW deployments at farms is the same as that already in use for standard agronomic practices, reducing barriers to implementation.
Subsidies and incentive structures for farming operations to implement specific practices are commonplace in most developed countries, and increasingly in developing countries. Agriculture is already a system with extensive subsidy structures for encouraging CDR, via no-till agriculture to facilitate soil organic carbon storage – though currently, agricultural systems are a large net source of greenhouse gases (GHGs) to the atmosphere (Rosa and Gabrielli, 2023). A significant source of agricultural emissions3 comes from the production/application of crushed carbonate rock (e.g., limestone) to raise soil pH via liming in settings where acidity is supplied by nitrogen fertilisers. Weathering of silicate rock can possibly replace liming as it will also increase pH. In addition to deacidification, applying rock as a mineral fertiliser may provide co-benefits to farmers by supplying plant-available micronutrients to soils that are otherwise scarce in many depleted systems (Beerling et al., 2018; Haque et al., 2019; Vienne et al., 2022). Besides the potential to contribute significantly to net-zero greenhouse gas emissions goals, EW has been shown to increase water infiltration and holding capacity as additional potential agronomical benefits (e.g. Beerling et al., 2024). A recent study in a Kenyan smallholder system showed that EW boosted maize yields by over 70 %, improved soil fertility, and increased micronutrient availability (Haque et al., 2025). This study demonstrates that EW is not merely a theoretical carbon removal strategy but a practical agricultural intervention with the potential to significantly improve livelihoods in some of the world's most vulnerable regions. For this reason, application of basalt and other mafic rocks to fields is an agronomic practice that has been carried out for centuries around the world, albeit at relatively small scale compared to modern farming methods (Swoboda et al., 2022). Overall, techno-economic and life-cycle assessments of EW deployments in various geographies are generally favourable to the feasibility of EW to scale as a method of CDR (Renforth, 2012; Beerling et al., 2020; Kantzas et al., 2022; Zhang et al., 2023).
To allow CDR methods to scale rapidly but robustly, it is important to develop relatively simple monitoring, reporting and verification (MRV) approaches that are sufficiently rigorous to give a high degree of confidence in the veracity of carbon removal. A variety of MRV approaches are currently being developed, which either focus on quantifying weathering products primarily in the aqueous phase but also secondary solid phases, or using soil-based approaches to estimate the fraction of rock powder that has dissolved (Almaraz et al., 2022; Clarkson et al., 2024). Most, if not all, aqueous-phase-based protocols are based on inorganic carbon accounting. This is due in part because of the requirement of permanent carbon storage, but mostly because of the assumption that the inorganic and organic carbon cycles in soils can be treated as two distinct systems.
Recently, several publications have stressed the importance of the organic carbon cycle on the net impact of CDR via EW, and suggested a requirement to include the organic carbon pool into the MRV of EW (Klemme et al., 2022; Vicca et al., 2022; Yan et al., 2023; Linke et al., 2024a, b; Manning et al., 2024; Niron et al., 2024; Vienne et al., 2024, 2025; Anthony et al., 2025; Lei et al., 2025). Organic carbon fluxes are easily 10× higher than the inorganic carbon fluxes generated through EW. Because soils contain substantial amounts of organic carbon (SOC) it is critical to avoid SOC loss as much as possible or compensate them by the gain in soil inorganic carbon (SIC). High efficiency crop production is desirable, where as much biomass per acre is grown in the shortest possible time. As a consequence of root respiration, the production of humic substances, intense microbial respiration and fertilizer oxidation, soil waters become more acidic. The protons of dissociating organic or non-carbon inorganic acids may protonate bicarbonate, thereby shifting the carbonate equilibrium towards a degassing of CO2. The decomposition of organic matter not only releases CO2, but is also an important step in the formation of so called “Mineral Associated Organic Matter” (MAOM) as the negative charge of the remaining organic carbon will retain the basic cations via charge balance. Overall, these processes may stimulate further weathering but also delay the CDR. It is the balance between the release of basic cations, the production or organic acids and the decomposition of organic matter that control the shift in pH.
However, carbon accounting in an open system such as arable land is extremely difficult because of the efflux of CO2 to the atmosphere and the unknown impact of “organic alkalinity” on the carbonate chemistry in the pore water. In a recent publication te Pas et al. (2025) conclude, on the basis of a short term soil column study, that 93 % to 98 % of the weathering products are retained within the soil profile and do not show up in leachate. This is in line with several other experiments that reported no or low leaching losses (Larkin et al., 2022; Vienne et al., 2024, 2025) and modelling studies suggesting lag times of up to several decades (Kanzaki et al., 2024). Hence, leachate-based total alkalinity (TA) measurements hugely underestimate weathering rates. te Pas et al. (2025) argue that soil-based mass balance approaches are more useful in quantifying weathering rates. Here, we build on the idea that cation accounting (Reershemius et al., 2023; Clarkson et al., 2024; Suhrhoff et al., 2024; Vienne et al., 2025) is a more practical and cost-effective MRV strategy, and equally as informative as accounting for all carbon species (summarized in Fig. 1).
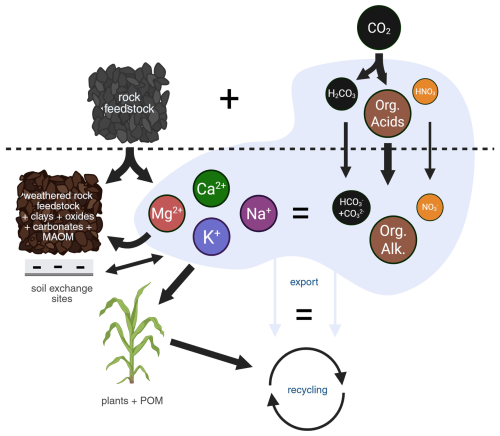
Figure 1Cations released from rock feedstock (cation alkalinity) is balanced by the sum of carbonate alkalinity, organic alkalinity.and e.g. nitrate from fertilizer nitrification. The relative contribution of the acids is indicated by the thickness of the arrows. Note that their relative importance can vary in different soils and under different conditions. Most of the cations will be incorporated into the crop and clay minerals. The latter stabilize soil organic matter as a transient pool of MAOM. Created in BioRender (Reershemius, 2025).
Alkalinity in the ocean can be expressed in terms of a cation surplus (i.e. cation “currency”) or in terms of the anion gap that is filled by negatively charged carbonate species (i.e. carbon “currency”). Importantly, in the ocean both “currencies” have the same value but alkalinity in the ocean is traditionally measured and expressed in carbon “currency”, using a (Gran) titration method and Dickson standards. This is attractive as it is expressed in the same currency used for the carbon credit market and therefore it has been the preferred currency also for pore water chemistry when monitoring EW in arable soils.
Formally, ocean TA is defined as the excess of proton acceptors over proton donors (see Appendix B). The same definition can be applied to pore water in soils, but the outcome of the standard alkalinity titration is different because of differences in the chemical composition of soil pore water compared to seawater. TA in seawater is dominated by carbonate alkalinity (). Hence, for ocean water the outcome of Dickson's alkalinity titration almost equals carbonate alkalinity which is almost equivalent to TA.
While for ocean water TA can be used in combination with either DIC or pH to calculate , this is not the case for pore waters in the terrestrial realm, where variations in pore waters are dominated by the organic carbon cycle in the soil (as indicated by the high pCO2 values). Song et al. (2023) found in coastal waters that “OrgAlk might modify H+ concentrations by 3 %–69 % (i.e., pH by 0.01–0.78)” and this may be even more pronounced in arable soils and thus also compromise pore water pH. Hence, using TA and pH to calculate in leachate as a proxy for EW, will produce erroneous numbers.
Organic acids introduce other acid-base species that contribute to alkalinity. This implies that TA, as determined via titration, is not equal to “carbonate alkalinity” and can therefore not be used to calculate other carbonate system parameters.
In the terrestrial system, the soil matrix keeps the organic biomass and the decomposition thereof to the site of production and only dissolved inorganic (DIC) and organic carbon (DOC) will enter the groundwater. As a result of intense microbial respiration of particulate organic matter (POM) and the soil pore structure, soil pCO2 is much higher than in the atmosphere (up to 4000–40 000 ppm; Dietzen and Rosing, 2023) leading to a significant efflux into the atmosphere (Paessler et al., 2024, 2025)4, while the remaining POM resides in the topsoil. Many of those organic molecules are negatively charged and thus contribute to an “organic alkalinity” pool balancing cations, which as a first estimate can contribute 82.5–137.5 µeq L−1 to TA in the standard titration procedure (see Appendix C).
There is also growing consensus that dissolved organic matter (DOM) can significantly contribute to TA in coastal waters (Hemond, 1990; Cai et al., 1998; Ulfsbo et al., 2015; Kerr et al., 2021, 2023; Wang and Cai, 2025). Ulfsbo et al. (2015) show that the measured excess alkalinity in the Baltic is consistent with an organic alkalinity derived from dissolved organic carbon, assuming that this dissolved organic carbon consists entirely of terrestrial humic substances. The contribution of polydisperse materials such as humic substances to titration alkalinity complicates Dickson's operational definition of titration alkalinity. In principle, organic acids could be accommodated by Dickson's TA definition if the pK values of these acids are known. The problem is that we usually do not know which organic acids contribute to TA and that their pK values are not discrete as for the carbonate or borate systems but are rather a polydisperse distribution of many pK values (Ulfsbo et al., 2015). It is therefore concluded that titration alkalinity should currently not be one of the parameters used to characterise the CO2 system in organic-rich waters, such as pore waters from agricultural fields. The organic alkalinity, e.g. in the Baltic, typically originates from riverine and groundwater fluxes derived from such fields. As mentioned before, this is not surprising because agricultural fields can be viewed as organic carbon reactors where, next to above ground biomass production, charged organic molecules such as e.g. humic acids are produced and microbial decomposition and humification produce charged POM and DOM. Consequently, the contribution of organic alkalinity can be significant and needs to be considered for carbon based MRV of EW in agricultural settings. To account for this, methods have been developed to quantify organic alkalinity directly (Kerr et al., 2023; Song et al., 2023; Wang and Cai, 2025). However, these methods are currently not used in carbon based MRV protocols (such as those proposed by Isometric (https://registry.isometric.com/protocol/enhanced-weathering-agriculture/1.1, last access: 18 December 2025) and puro (https://puro.earth/puro-standard-carbon-removal-credits, last access: 18 December 2025)).
For carbon accounting, usually two of three parameters (DIC, pH and alkalinity) are determined in the leachate to calculate flux, the target parameter with regard to permanent carbon storage in the ocean. However, the presence of a significantly negatively charged organic carbon pool, not only renders the titration method ineffective (as it does not result in the quantification of carbonate alkalinity) but also compromises pH (Song et al., 2023). Because of the high pCO2 in pore waters, it is hardly possible to extract a sample for DIC analysis without equilibration to ambient conditions. As a consequence of the change in pH, and cannot be calculated with any significance.
As life is carbon-based, the DIC leaving the field, entering the groundwater and the river will be recycled many times in the food-chain before it finally enters the ocean, probably much later than the cations. Hence, the additional carbon finally stored in the ocean due to EW is almost certainly not the bicarbonate from the leachate in the field, but pre-existing DIC in the acidified ocean that is charge-balanced by the cations from rock weathering on the field. Importantly, the cation flux to the ocean will drive the same shift in radiative forcing as the equivalent removal of CO2 via the production of bicarbonate on the field.
The addition of rock-flour to a soil leads to the release of cations that affect the chemistry and the biology of the soil. Hence, rock amendments may directly or indirectly affect soil fauna, which are known to play an important role in regulating SOM decomposition and stabilization. Changes in the activity or abundance of key soil ecosystem engineers, such as earthworms, but also other macro- and meso-decomposer groups, could have long-term and unpredictable consequences on SOM stabilization (Lubbers et al., 2013; Zhang et al., 2013).
Earthworms are often regarded as key ecosystem engineers due to their capacity to ingest, fragment, mix and transport inorganic and organic matter, making their feeding and burrowing behaviour a key ecological property (Vidal et al., 2023). The optimal pH for earthworms ranges between 6 and 8 (Hou et al., 2005). Yet, no changes in the survival of deep-burrowing (e.g. Lumbricus terrestris) or shallow-burrowing (e.g. Aporrectodea caliginosa) earthworms, both naturally occurring in the basaltic soils on Iceland (Sigurdsson and Gudleifsson, 2013), were observed after basalt amendment, even though the pH in basalt amended mesocosms rose up to circa 8.5 (Vienne et al., 2024). In artificial organo-mineral systems, the survival and activity of the shallow-burrowing species Apporectodea calignosa and Allolobophora chlorotica, depended mostly on variables influencing the structure and drainage potential of these systems (Calogiuri et al., 2025b). These authors also found that surviving earthworms had a neutral or negative effect on weathering indicators in their artificial organo-mineral mixtures, which they attributed to the short length of their experiments or changes in organic rather than inorganic carbon concentration. In contrast, dead earthworms enhanced almost all weathering indicators considered, which was suggested to be the result of microbial processes stimulated via decomposing earthworm bodies. These results were recently confirmed by Calogiuri et al. (2025a), who showed that while live earthworms directly stimulated the formation of mineral-associated organic matter (MAOM), dead earthworms stimulated microbial activity, which enhanced both MAOM formation and inorganic carbon capture.
Interestingly, Needham et al. (2006) showed that the ingestion of unweathered basalt from Iceland by the common marine lugworm Arenicola marina, had no detrimental impact on the worms but that weathering rate was one hundred to one thousand times faster than in experiments of standard, inorganic basaltic weathering. They concluded that sediment ingestion and the entire coprophagic cycle are highly significant for sediment alteration, early diagenesis and the origin of clay minerals in sedimentary rocks.
Besides an impact on soil fauna, addition of rock-flour also affects the dynamics of both the organic and the inorganic carbon fluxes between the different pools will change, which may lead to an increased carbon storage in long-term reservoirs, as also seen for liming in mineral soils (Wang et al., 2021; Zhang et al., 2022).
Soil mineral amendments for enhanced weathering alter key soil properties such as pH and nutrient availability, stimulating microbial activity, and thus organic matter decomposition and SOC dynamics (Vicca et al., 2022; Buss et al., 2024). For example, Yan et al. (2023) observed that wollastonite addition significantly increased soil pH and DOC, leading to a 330 % increase in soil CO2 efflux across 12 land-use types (see also Paessler et al., 2024, 2025). Xu et al. (2024) reported raised pH and microbial activity, with corresponding increases in soil enzyme activities and CO2 emissions, after basalt application. Surprisingly, the CO2 fluxes to the atmosphere and the increase of alkalinity in the leachate was more dependent on soil type than on the feedstock itself, suggesting a strong control by biology and soil composition (Paessler et al., 2024; A notable exception is steel slag, probably because it is extremely reactive).
On the other hand, a major outstanding question is whether increased CO2 efflux simultaneously increases the residence time of the remaining SOC as a result of soil mineral amendments. Increases in MAOM have been reported (e.g. Bai and Cotrufo, 2022; Chen et al., 2024). In recent papers (Ramos et al., 2024; Steinwidder et al., 2025) the relationship between silicate weathering and organic carbon burial is discussed and concluded that clay mineral formation is the principal modulator of weathering fluxes and OC accumulation in soil. Hence, silicate addition may also stimulate SOC stabilization. Buss et al. (2024) also emphasized the role of primary silicates, such as basalt, in promoting SOC stabilization by forming MAOM and macroaggregates. In line with this, Vienne et al. (2024) observed reduced decomposition and CO2 release following basalt application, but this effect depended on the presence of earthworms. Through cation tracing, Niron et al. (2024) found that basalt amendments enhanced the formation of secondary minerals such as clays and oxides. They also observed a reduction of the soil CO2 efflux by approximately 2 t CO2 ha−1, suggesting stabilization of SOM.
Increases and decreases in organic matter decomposition with mineral amendments have been reported, probably caused by soil type and properties, plant activity, microbial communities and interactions, indicating the context-dependency of the processes involved. Moreover, short-term effects on organic matter decomposition may be completely different from the long-term impact. Emerging evidence indicates that EW influences organic matter stabilization not only through added reactive minerals but also via changes in microbial activity (Boito et al., 2025; Sohng et al., 2025). Silicate amendments may initially stimulate SOM decomposition (as is common when pH increases), yet under some conditions may promote MAOM formation (Steinwidder et al., 2025). These responses are strongly context-dependent, and decreases in MAOM formation have also been observed (Sokol et al., 2024). Sohng et al. (2025) demonstrate the importance of quantifying trade-offs between the co-application of silicate and organic amendments on the fate of SOC stabilization. It is critical to explicitly integrate biotic and organic processes into EW assessments (Boito et al., 2025). Ultimately, the EW effect on SOC stocks will reflect a balance between shorter-term destabilization and longer-term stabilization of SOM through MAOM.
Only few experiments have explored the impact of EW on SOC dynamics, and these experiments were typically short in duration (weeks or months). However, time is expected to play a crucial role in shaping the balance between decomposition and stabilization. Stabilization effects, particularly through the formation of MAOM, may become increasingly important over time as mineral weathering progresses (Vicca et al., 2022).
Closing budgets may be very difficult in open systems such as arable land and extremely cumbersome and expensive. Budgets can be carbon based (i.e. the “true” currency for carbon credits), or based on cation accounting. As stated before, both can be viewed as a different currency for alkalinity but are interchangeable. In the ocean, where the carbon is finally and permanently stored, the ratio is roughly5 equal to one (one mol carbon for one mol cation charge), although carbonate buffering likely reduces this to 0.7–0.9 (Renforth and Henderson, 2017).
For porewaters on land the situation is completely different and is expected to be strongly context-dependent. Continuously monitoring all of the very dynamic organic and inorganic carbon sources and sinks in such an open system, especially in the first few years, is very difficult and expensive as it requires continuous monitoring. Therefore, it may be almost impossible to close the budget. Uncompetitively high requirements and costs for carbon based MRV compared to other approaches may impede the upscaling of EW. Here we argue that the MRV on the basis of cation accounting is more practical and efficient, complemented by monitoring SOC.
We propose a cation-based MRV that is related to the solid phase as it is much easier to handle in an open system and does not require continuous monitoring of carbon leaving the soil as biomass, in leachate or as a flux into the atmosphere. The “explicit conservative expression for total alkalinity” formulated by Wolf-Gladrow et al. (2007) is ocean-based but can easily be adopted for farmland and related to the biogeochemical processes in agriculture (e.g. impact of fertiliser/manure and liming).
Both TA and DIC are conservative quantities with respect to mixing, changes in temperature and pressure and are therefore used in (oceanic) carbon cycle models as prognostic parameters. For both the ocean and terrestrial (pore)waters it is thus important to understand changes in TA due to various biogeochemical processes.
It is virtually impossible to disentangle the impact of processes such as liming (dissolution and precipitation of carbonate) or the formation and remineralization of organic matter (what agriculture is actually about) or the addition of fertiliser or manure from the common expression for TA in terms of concentrations of non-conservative chemical species (, , , H+, OH−, etc.). Hence, Wolf-Gladrow et al. (2007) derived an expression for explicitly conservative total alkalinity (TAec; see Appendix B) in terms of the total concentrations of major ions (Na+, Cl−, Ca2+ etc.), and the total concentrations of various acid-base species (total phosphate etc.) from Dickson's original expression of TA under the constraint of electroneutrality. Changes in TA by various biogeochemical processes are then easily derived from the “explicit conservative expression” for TA because each term in this expression is conservative and obeys a linear mixing relation (Soetaert et al., 2007; Middelburg et al., 2020). In the open ocean, all cations released from dissolving feedstock would be exactly equivalent to the carbonate alkalinity as determined by Dickson's titration. As explained in Sect. 3, it is due to differences in matrix (water vs. soil) that organic matter is efficiently transferred through the food chain to below the mixed layer of the ocean but mostly remains in the top soil in terrestrial systems where it is broken down into POM and DOM creating a mismatch between titration (“carbonate”) alkalinity and cation alkalinity, mainly due to the presence of organic alkalinity. For determining the CDR in soils, we therefore propose soil-based mass-balance approach (e.g. Reershemius et al., 2023). In principle, all cations released from dissolving feedstock in agricultural soil are potential alkalinity to neutralize dissolved carbon in the ocean. Their fate is discussed in more detail in Sect. 6.
In practical terms, the method proposed by Reershemius et al. (2023) for cation budgeting can be used in the field, provided soil heterogeneity in cation concentration can be accounted for to detect both feedstock addition and dissolution at relevant signal to noise ratios (Suhrhoff et al., 2024). A big advantage is that continuous sampling is not required but intervals of one or two years and can be synchronised with the sampling that farmers carry out to analyse soil parameters such as nitrate and soil pH. Below the depth of typical soil sampling, the budgeting for cations can only be done via models, just as for carbon. Once the “cations” reach the ocean, the “cation currency” can be exchanged for the true “carbon currency” of carbon credits at an “exchange rate” of roughly one mol carbon for each mol of charge using Wolf-Gladrow et al. (2007). A suitable efficiency factor could be attributed to account for losses associated with carbonate buffering potentially between 9 %–30 % (Renforth and Henderson, 2017; Kanzaki et al., 2023; Zhang et al., 2025).
The cation exchange capacity (CEC) of a soil represents the total quantity of cations that can be reversibly adsorbed onto negatively charged surfaces, such as clay minerals and organic matter. These exchange sites can be occupied by both basic cations (Ca2+, Mg2+, K+, Na+) and acidic cations (H+, Al3+, Fe3+, ). While the cation incorporation into secondary clay minerals is mostly permanent due to isomorphic substitution and thus fixed, the CEC contributed by organic molecules increases with pH due to deprotonation of their functional groups (Blume et al., 2016; Weil and Brady, 2017). The base saturation (BS) reflects the fraction of the CEC occupied by base cations. Higher BS generally corresponds to higher soil pH, as these cations neutralize acidity. Together, soil pH, CEC, and base saturation control the mobility and availability of cations, influencing mineral weathering and the formation of secondary clays. These processes, in turn affect both the fate of cations and carbon sequestration in enhanced weathering systems.
The majority of the cations from the dissolving feedstock end up as clays in the field and a small fraction of those in-situ neo-formed clays will be eroded downstream and finally end up in the ocean. However, this effect is entirely unquantified, and will vary hugely with location. Hence, the primary process of CO2 sequestration by ERW is the clay-organic carbon interaction of neo-formed clays and the primary research question for the efficacy of EW boils down to how much carbon is associated with clays (mol C per mol cation)?
Clay minerals transported via rivers to the ocean also bind organic carbon and when entering the ocean, organic rich deposits are formed in estuaries via a process called “flocculation”. Unfortunately, it is difficult to assess the potential for the relative impact of CO2 sequestration by secondary clay-organic interaction in the ocean vs. the impact of cation sequestration by in-situ clay formation in the soil system and binding SOC. The strong relationship between organic matter and mineral surface area in recent and ancient marine sediments suggests that adsorption of carbon compounds onto clay mineral surfaces played and plays a fundamental role in the burial and preservation of organic carbon (Hemingway et al., 2019; Kennedy and Wagner, 2011; Kennedy et al., 2002, 2014).
Cations released by rock weathering end up in many different fractions, mostly associated with a temporal/transient storage of carbon, primarily due to pH-dependent negatively charged exchange sites provided by the cation exchange capacity (Dietzen and Rosing, 2023; Zhang et al., 2024). As a result, most cations are temporarily held back before they reach the “depth of no return”. At some point, cations are expected to start leaking through (in geochemical terms when the “breakthrough” is reached). The cation-flow will most likely always be retarded, but the breakthrough time of the system may vary as more cations are added on top depending on e.g. soil properties, rock characteristics, secondary mineral formation etc. It seems likely that the soil's cation “capacitor” also becomes saturated. The composition of the cations leaving the “capacitor” are very likely dependent on pH and water flow. Kanzaki et al. (2024) argue that the temporal disconnect between deployment of EW and climate-relevant CDR may have significant implications for the integration of EW into (voluntary) carbon markets. The question therefore is, if only those cations should be accounted as a “carbon credit” that will directly and/or finally and permanently neutralize bicarbonate in the ocean? In the following we discuss the different pathways that cations can take, how they can also stabilize carbon on climate relevant timescales in transient SOC pools, and what the implications of cation loss for carbon fluxes are. The impact of agricultural practices, such as liming and fertilisation, on cation dynamics are briefly discussed in Appendix D.
6.1 Element exchange
Both soil organic matter as well as clay minerals contribute to ion exchange and will be discussed below in separate sections. Soils have both an anion exchange capacity (AEC), and a cation exchange capacity (CEC) (e.g. Bergaya et al., 2013). The exchange capacity adsorbs anions or cations onto mineral surfaces (particularly clays or oxides), but also allows the continuous exchange with other negatively or positively charged ions in the soil pore water. In most soils, the CEC is much greater than the AEC, but this can change in highly weathered soils (Weil and Brady, 2017). CEC comprises both a permanent part (due to isomorphic substitution in 2:1 phyllosilicate minerals) and a pH-dependent part composed of functional groups on edges of clay minerals and oxides and functional groups on organic matter. Under low pH conditions6, the pH-dependent CEC will decrease as protons neutralise the negative charge of functional groups such that the AEC may become dominant in the pH-dependent part.
From the perspective of enhanced weathering, the ions (of both charges) liberated during silicate weathering (including atmospheric-derived bicarbonate), will at first be taken up by the soils' exchange capacity. This may, depending on “base saturation” and “spare capacity” also cause preexisting ions to desorb. These will then re-adsorb further down in the soil, causing a cascade reaction that retards the breakthrough of both anions and cations to groundwater, or indeed the pore water measuring point. Observationally, this process can take several years, and will depend on the soil characteristics (e.g. amount of clay), pore water pH, and water flow rates. Breakthrough times will also vary according to element (e.g. Pogge Von Strandmann et al., 2019). These downward exchange reactions will likely still occur even when the CEC is “filled” with amendment-derived ions, so that new ions will always be retarded in their arrival, which could create some issues in a solution cation-based MRV method. For equal molar concentrations ion sorption preferentially occurs in the order: (and desorption vice versa), but the preferential ion sorption order varies for different clay minerals, is dependent on the charge density (lyotropic (Hofmeister) series), depends on the absolute concentration and is pH dependent (e.g. Farrah et al., 1980; Gislason et al., 1996; Doi et al., 2020; Kunz et al., 2004).
As mentioned above, the CEC complex is not composed purely of inorganic clays and cations will also adsorb to organic compounds such as humic substances. In fact, depending on pH, humic substances account for 50 %–90 % of the CEC in soils. The definition of humic substances is largely operational and is rooted in the history of soil science (Stevenson, 1994). “Humic substances” is an umbrella term covering humic acid, fulvic acid, humin, hydrophilic acid, etc. In terms of chemistry, they represent a spectrum of humic molecules, constructed from similar (poly)aromatic, aliphatic, and carbohydrate units and containing the same functional groups (mainly carboxylic, phenolic, and ester groups), albeit in varying proportions (Ghabbour and Davies, 2001). The presence of carboxylate and phenolate groups gives these substances the ability to form chelate complexes with ions such as Mg2+, Ca2+, Fe2+, and Fe3+ (Tipping, 1994), which is an important aspect of the biological role of humic acids in regulating bioavailability of metal ions to crops. The presence of carboxylic and phenolic groups gives these substances the ability to form chelate complexes with ions such as Mg2+, Ca2+, Fe2+, and Fe3+ (Tipping, 1994), which is an important aspect of the biological role of humic acids in regulating bioavailability of metal ions to crops. Chelation is also important to speed up weathering, as it avoids secondary mineral precipitation at the mineral surface. The chelates release the cations lower in the soil profile, potentially leading to podzol formation.
When relating cation to carbon fluxes, it should be assumed that cations that are lost from solution to exchangeable sites will cause a charge-equivalent loss of bicarbonate due to re-equilibrium of the carbonic acid system (Kanzaki et al., 2024). If base cations adsorption is charge balanced by a release of protons (i.e., increase in base saturation), the resulting released acidity may react with bicarbonate and cause degassing of CO2. The CDR associated with the cations lost to the CEC complex may be realized at a later point in time when base cations are released from CEC sites due to a decrease in base saturation (Kanzaki et al., 2024).
6.2 Clay mineral formation
Secondary minerals that form from silicate weathering (oxides, clays, zeolites) are ubiquitous during weathering. Initially, rainwater is undersaturated with regard to primary silicate minerals (olivine, pyroxene, feldspar, etc.). However, the dissolution of these minerals drives secondary minerals to supersaturation, and their precipitation then maintains primary mineral undersaturation, which allows these minerals to carry on dissolving. Rainwater is initially undersaturated for all silicate minerals (primary and secondary), so the first step of dissolution is “for free”, but then secondary minerals will also start precipitating. Secondary mineral formation may have direct effects on enhanced weathering processes, such as a physical change of field drainage patterns or surface passivation, where the relatively unreactive secondary minerals coat primary mineral grains, slowing reaction rates (e.g. Beerling et al., 2020; Calabrese et al., 2022). Secondary minerals also cause chemical changes. Principle among these is the effect secondary minerals have on CO2 sequestration. Simply put, secondary minerals can retain cations that would otherwise be used to sequester CO2 by balancing carbonate or bicarbonate ions in pore waters. Thus, at first glance, secondary mineral formation seems to make CO2 sequestration via silicate weathering less efficient; or, to put it another way, clay formation is a source of CO2 (e.g. Pogge Von Strandmann and Henderson, 2015; Kalderon-Asael et al., 2021; Pogge Von Strandmann et al., 2021). However, algae in soils and streams preferentially leach nutrients from secondary minerals (Grimm et al., 2019), and this attachment to clay particles therefore helps to bury the associated organic carbon (Kennedy and Wagner, 2011; Kennedy et al., 2014). When fresh water enters the ocean, flocculation of clays and associated organic matter occurs. Thus, while clays hinder CO2 sequestration via the inorganic pathway which is the primary goal of ERW, they may assist CO2 sequestration via the formation and burial of organic carbon.
When observing element mobility in basaltic weathering, K and Na mostly stay in solution while, e.g. Fe and Mn mostly go into secondary minerals. For example, in a basanite from the Eifel region, Ca and Mg have mobilities around 5× less than Na, suggesting that 80 % of Ca and Mg are going into clays. In basaltic rivers, about 70 % of Ca is going into clays. Figure 2 summarizes estimates of the proportion of Ca and Mg from weathered basalt ending up in secondary precipitates (notably clay minerals) from several studies. It shows that the percentage of cations retained by secondary mineralisation is highly variable but a dominant process that needs to be taken into account with regard to the MRV of EW.
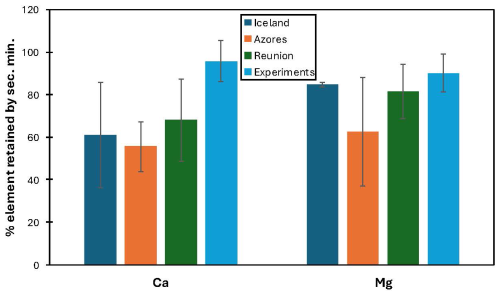
Figure 2Estimates of the proportion of Ca and Mg from weathered basalt ending up in secondary precipitates (notably clay minerals), for Iceland, Azores (Pogge Von Strandmann et al., 2010), Reunion (Louvat and Allègre, 1997). The basalt weathering experiments are from Pogge von Strandmann et al. (2019). The overall method of the mobility calculation comes from Gislason et al. (1996). The error bars are the variability in the different rivers.
Amorphous precursors of clay minerals, such as imogolite (Al2SiO3(OH)4) and allophane (), form very quickly and are characterised by a large specific surface area with variable and permanent surface charge, having a great affinity for organic functional groups. Basile-Doelsch et al. (2007) show that these amorphous precursors can store large amounts of organic matter which turns over very slowly and plays a pivotal role in the storage of a transient organic carbon pool on geological timescales beyond 100 ky.
Hence, secondary minerals contribute significantly to the stabilisation and build-up of SOC in the form of MAOM. Recent work (Niron et al., 2024; Vienne et al., 2025; Steinwidder et al., 2025) demonstrates that sequential extraction methods are powerful tools to understand the role of cations and secondary mineral formation in SOC stabilisation. Some conditions (e.g. high pH) will favor carbonate formation, while other conditions, such as low water flow rate lead to oversaturation and clay formation or cation bridging with SOC (Rowley et al., 2018; depending also on the type of OM). Song et al. (2022) conclude that while Fe minerals (e.g. pyroxenes) play an important role in stabilizing SOC, the electron transfer role of Fe also exerts a crucial influence upon SOC turnover. For instance, the coupling between Fe3+ reduction with SOC oxidation can increase the efflux of CO2.
In many enhanced weathering studies secondary mineral formation has been neglected, as its formation rate was considered to be too slow and measuring clay formation (as opposed to clay presence) has traditionally been difficult. “Non-traditional” isotopes, such as those of lithium or magnesium, can however measure and quantify secondary mineral formation (e.g. Pogge Von Strandmann et al., 2021). Using these methods, studies have shown that clay formation is often rapid, with experimental studies showing clay formation within a few hours (Jones et al., 2012; Pogge Von Strandmann et al., 2019, 2022a, b, 2025) and natural groundwater studies showing clay formation within 1–2 months (Oelkers et al., 2019). This can be understood as primary silicate minerals cannot continue to dissolve without the reaction products being washed away or incorporated into secondary minerals.
However, the amount of secondary mineral formation per unit of silicate rock dissolved varies according to different parameters. Natural river studies have shown under which conditions secondary mineral formation is maximised. This largely depends on the water-rock contact time, which in turn is controlled by the discharge (e.g. Dellinger et al., 2015; Wilson et al., 2021; Zhang et al., 2022; Pogge Von Strandmann et al., 2023), and “shielding” by previously formed secondary minerals (Krause et al., 2023). Thus, when discharge is high, water-rock contact times are low, and secondary mineral formation is minimised. Conversely, when discharge is low, secondary mineral formation is relatively high. This means that in rapidly draining soils with a high porosity, the CO2 drawdown efficiency by silicate weathering may be higher. Repeated amendment with silicates may change the porosity however, and thus diminish the CO2 sequestration efficiency. In conclusion, cations stored in secondary minerals cause an equivalent loss of CO2. The timescale over which this happens and the fraction of loss is variable (and can therefore be optimized for).
6.3 Soil Organic Carbon (SOC) and Dissolved Organic Carbon (DOC)
Potential increases in SOC are a bonus on top of inorganic CDR – but a very interesting one, as the stabilisation of SOC via cation-bridging (e.g. Rowley et al., 2018) and association with minerals (MAOM; Chen et al., 2024), stores ca. 5 (Matus et al., 2014) to 10 times (Bai and Cotrufo, 2022) more carbon per unit charge/alkalinity than bicarbonate. Chen et al. (2024) call this the mineral carbon pump. MAOM may be stable for centuries but is sensitive to land use and mineral type (Bramble et al., 2025) and to climate change (Wu et al., 2025). However, since it is a transient storage and to use a conservative accounting, those cations should only be counted as “one charge finally balancing one bicarbonate ion”, just as cations reaching the ocean. When MAOM is decomposed, a small part of the cations may or may not end up in bacterial necromass. The elemental composition of microbial biomass may vary between C4H7O1,5N to C5H8O0,8N with small amounts of P and S and trace amounts of Fe, K, Mg, Ca (Kästner et al., 2021). It is therefore safe to argue that the ratio of carbon per unit charge is much larger than unity and therefore much higher than for the final storage of carbon in oceanic carbonate alkalinity. Note that the remainder of the respired cations is not lost, but free to, either balance bicarbonate or to stabilise fresh SOC again. The bottom line is that the carbon/charge ratio during transient storage will not go below unity as in the final permanent storage in the ocean and therefore that SOC should be accounted for on the basis of one mol carbon for one mol of cation charge.
6.4 The crop
Part of the cations will be taken up as micronutrients by the crop (e.g. Zn, Si, Fe, etc.) and potentially contribute to productivity and increased resistance to drought, frost, disease, and pests. Base cation charges in the crop are probably small in comparison to the soil.
If the crop is e.g. fodder for cattle, most of the cations will be returned to the (same or another) field as manure (minus a small percentage remaining in the cattle). If the crop (or the beef) is for human consumption, most of those cations will quickly end up in sewage. Assuming that the cations are not retained in the sewage treatment plant, most of the cations will be released to a river and finally reach the ocean even before those taking the route via the groundwater from the fields. Hence in terms of carbon removal potential of EW, these cations are also still “in the game” and should be accounted for in the MRV provided the considered timescale is long enough for biomass to be decomposed and for the contained cations to once more charge balance bicarbonate.
Precise accounting may be difficult but in practice could be calculated as a percentage, reflecting the conversion efficiency of the food chain, i.e. the relative amounts of cations remaining in the organism (cattle, pork, chicken, etc.). For other products such as oilseed rape, similar calculations can be imagined, but finally most of the food produced will go through human stomachs or end up as compost which will finally be returned to the soil. Vienne et al. (2025) report that base cation charges in the plant pool were about two orders of magnitude smaller than in the soil pool (for maize plants) suggesting that the cation loss to the crop, and to the subsequent food chain, can therefore be neglected in the balance.
It is important to realise that the organic and the inorganic carbon cycles in agricultural fields are intimately related and cannot be separated for the MRV of EW. The idea that only the bicarbonate flux should be accounted for with regard to a carbon credit system is wrong as the impact of EW on a transient SOC pool can be significant and is stable.
It is correct to use total alkalinity (TA) as a master variable to monitor the efficacy of EW but the titration method that is currently applied to leachate waters to calculate the bicarbonate concentration is not suited as it does not include organic alkalinity. Furthermore, analysing and monitoring the carbon flows to close the budget is very hard to realise in an open system and requires continuous monitoring.
We propose to apply the explicit conservative expression for TA (TAec) in terms of the total concentrations of the major ions. The association of a major part of the cations with soil organic carbon via clay minerals may be transient but the stabilisation and storage is on climate-relevant timescales and just as effective as balancing DIC in the ocean. The carbon sequestration per cation charge is higher for organic- than for the inorganic-pathway. Even though most of the carbon associated with the additional cations from EW will finally be respired, the cations will stabilize fresh SOC and/or end up balancing carbonic acid or nitric acid from fertilisation. Hence, while the SOC pool is transient, the SOC concentration will likely increase and become higher than before EW.
Clay mineral formation can play a dominant role in the overall CDR by EW (Pogge Von Strandmann et al., 2025). A substantial fraction of the cations dissolving from the crushed rock may relatively quickly turn into clay mineral precursors, which can have a great affinity for organic functional groups. Hence, most of the weathering products are retained within the soil profile and do not show up in leachate. Although clay mineral formation reduces the CDR potential for carbonic acid, it likely does not lower the overall CDR. The association of clay minerals and their precursors with SOC can even store more carbon per unit charge, potentially creating a bonus.
We make the case that a cation-based MRV is much more practical and reliable than a carbon-based MRV. It does not require continuous monitoring and can be combined with the (bi)annual soil sampling that is carried out anyway. Because of its simplicity and economic benefits, it may be favourable for the upscaling of EW as a natural NET. For the cation-based MRV we propose to sample the soil once a year, following the method proposed by Reershemius et al. (2023). The maximum CDR potential through cation liberation from dissolving rock can be calculated by stoichiometric analysis of the feedstock. The loss of cations from this is minimal and the majority of the cations are available for CDR.
Minor CDR losses from EW can be attributed to cation loss to crops. When used as fodder, the cations will mostly be returned via manure but the loss should be subtracted from the carbon credits for the farmer as he/she sells the produce. Finally, it should be noted that variability on the plot scale will be averaged by the law of large numbers, when EW is scaled up.
For the overall MRV several follow-up research questions arise:
-
What is the average ratio of carbon per cation charge in clay minerals? How does that relate to the average annual increase in SOC?
-
What is the elemental composition of different crops? How much cations per hectare, in which stoichiometry, need to be subtracted from the CDR potential of the feedstock?
-
What happens to cations in sewage treatment plants? How much and in what stoichiometry are they retained?
On (geological) timescales much longer than a few thousand years, the atmospheric carbon dioxide concentration is controlled by the balance between CO2 sources (volcanic degassing, metamorphism, rock organic carbon oxidation and potentially carbonate weathering by sulfuric acid in mountain ranges) and sinks such as burial of organic matter and the burial of carbonate minerals. Both of these sinks are almost entirely driven by weathering, as it provides alkalinity (see Appendix B) and cations such as Ca2+ or Mg2+ for carbonic acid neutralisation and carbonate formation (Walker et al., 1981), as well as nutrients that fertilise the formation of organic carbon and clay minerals that help to bury that organic matter (Kennedy and Wagner, 2011; Grimm et al., 2019). The oceans and the hydrological cycle play a pivotal role in partitioning this CO2, as CO2 not only dissolves in water but also reacts with it to form bicarbonate () and carbonate ions (). More than 99 % of the dissolved inorganic carbon (DIC) in the ocean is in its ionic form. The reactivity of CO2 with water is a major reason why the total dissolved carbon content of the ocean (DIC) is ca. 60× larger than the total CO2 concentration in the atmosphere. Consequently, the carbonate chemistry of the ocean controls the pCO2 of the atmosphere. At steady state, atmospheric pCO2 is in equilibrium with the concentration of molecular dissolved CO2 (so called “aqueous CO2” or CO2aq) in the mixed layer of the ocean. This is formulated by Henry's Law. CO2aq itself is a function of ocean pH, which is controlled by the ratio of DIC/total alkalinity (TA) entering the oceans via rivers. The combined biological and solubility pumps of the world ocean resulted in an atmospheric pCO2 of ca. 280 ppm in the Holocene.
Alkalinity is added to the ocean via rivers that transport the weathering products of rock (cations). Today, about 0.5 to 1 Gt of CO2 is globally removed by silicate rock weathering, according to the generalised chemical formulae (Gaillardet et al., 1999; Moon et al., 2014; Brantley et al., 2023):
The magnitude of the alkalinity flux is determined by the silicate weathering feedback (Walker et al., 1981; Berner et al., 1983), mostly by the hydrological cycle, the temperature and by the “weatherability” of the Earth's surface. The latter is determined by a combination of factors that make silicate rocks susceptible to chemical weathering such as uplift rate, biological alteration, and the composition of rock exposed at the Earth's surface (Kump and Arthur, 1997; West et al., 2005; Brantley et al., 2023).
The chemical weathering response on Earth ranges between supply/transport-limitation or weathering/kinetic-limitation, where a limit on weathering is imposed by pCO2, temperature, and hydrology, irrespective of supply. While Earth as a whole can be considered to have a weathering-limited regime, there are many environments that approach supply limitation – characterised by low relief and with well-developed, compositionally mature soil profiles (West et al., 2005; Maher and Chamberlain, 2014; Isson et al., 2020), such as arable land.
In addition to input fluxes, oceans are the site of major organic and inorganic carbon export. For at least the last 500 million years in Earth history, calcification by organisms has led to the accumulation of carbonate sediments in the ocean. Because the dissolution of calcium carbonate is pressure- (i.e. depth-) dependent, carbonates are only preserved above the so-called “carbonate compensation depth” (or CCD). The deep ocean below the CCD is devoid of carbonate. As the residence time of calcium is much longer than the turnover time of the ocean (ca. 1000 years), it is uniformly distributed with depth. Hence, the depth of the CCD is only determined by the carbonate ion concentration. By net dissolving or building up sediments, i.e. shoaling or deepening of the CCD, the ocean can buffer carbon perturbations on timescales of a couple of ocean “over-turnings” i.e. a couple of thousand years. On these timescales, atmospheric pCO2 is controlled by the “Rain Ratio”, i.e. the vertical fluxes of organic carbon to calcite to the deep sea (Archer and Maier-Reimer, 1994). On timescales longer than the biosphere and surface ocean response, rock weathering and ocean alkalization will be the primary way anthropogenic CO2 will be consumed and stored, and ocean chemistry rebalanced over the next 50+ years (Archer et al., 2009).
The anthropogenic carbon emissions into the atmosphere are now exceeding the natural controls as the ocean mechanism (“carbonate compensation”) cannot keep pace with the anthropogenic perturbation. As a consequence, atmospheric pCO2 is steadily increasing with a seasonal modulation driven by the terrestrial biosphere, as impressively shown by the Mauna Loa pCO2 record that started in March 1958!
One of the most promising ways of taking CO2 out of the atmosphere is to add alkalinity to the ocean, either directly (“Ocean Alkalinity Enhancement” or OAE) or indirectly via river run-off (produced as a consequence of EW on land), provided that alkalinity enriched waters are in contact with the atmosphere long enough for carbon drawdown to occur.
The concept of alkalinity in relation to seawater has its roots in the late nineteenth century investigations by Tornøe and Dittmar (see: Dickson, 1992) and since then has almost exclusively been used in the context of the oceanic carbonate chemistry, for instance, with regard to the natural control of atmospheric CO2. Of the 6 parameters defining the carbonate system (DIC, CO2aq, , , pH and TA) only DIC (dissolved inorganic carbon) and TA (total alkalinity) are conservative, i.e. they do not change with temperature or pressure and obey a linear mixing relationship (Wolf-Gladrow et al., 2007).
In formal terms, TA is defined as the excess of proton acceptors over proton donors and the alkalinity equivalence point is where TA=0. TA of a water sample can thus be estimated as the amount of HCl that has to be added to reach the equivalence point. The pH at which proton donors exactly balance proton acceptors (TA=0) is defined as the so-called proton condition and denoted by pH0. The proton condition is different for different acid-base systems and the choice of a particular acid-base system therefore defines the so-called “zero level of protons” for a single set of related acid-base species. The overall proton condition defines the zero level of protons for each single acid-base system.
For SW, a mixture of acid-base systems, zero level of protons and the pH where TA=0 must be defined. Hence, a single pK value must be chosen which applies for all acid-base systems as a dividing point between proton acceptors and proton donors. This pK is denoted as pKzlp, the pH at zero level protons and is specified as follows: The chemical species which dominates (largest concentration compared to the other chemical species of the same acid-base system) at pH=pKzlp defines the zero level of protons species, acids with pK<pKzlp are proton donors, and bases formed from weak acids with pK>Kzlp are proton acceptors. Dickson (1981) chose pKzlp such that it is below pK1 of the carbonate chemistry and so that systems that are of no interest do not contribute to TA (this was achieved by choosing pKzlp above those of hydrogen sulphate (pK=2) and hydrogen fluoride (pKHF=3.2)), i.e. . pKzlp=4.5 was chosen to correspond roughly to the pH of the conventional alkalinity end-point, and is nearly in the middle of this range between 3.2 and 6.3.
In more simple terms and as an approximation, TA can be understood as the difference between the sum of the cations minus the anions from strong, i.e. fully dissociated, acids and bases in the ocean. If 1 kg of sea water evaporates, approximately 35 g of salt will remain. A charge balance of the remaining cations minus anions will show a slight excess of positive charge. As charge balance is always obeyed in sea water, this excess positive charge is balanced by the dissociation of weak acids and bases (see: Wolf-Gladrow et al., 2007). To determine the alkalinity in seawater, Dickson (1981) developed an operational definition using volumetric titration with a strong acid. By far the dominant contributor to alkalinity comes from deprotonated carbonic acid (“carbonate alkalinity”) and the remainder from deprotonated boric acid7 and a negligible small fraction from silicate and phosphate (“nutrient alkalinity”). The organic fraction of TA (OrgAlk, from organic acids/bases) is typically deemed negligible in the open ocean. Although it is not explicitly accounted for in conventional TA expressions, it is included by the ellipses (“…”) in the equation below that stand for additional, as yet unidentified, acid-base species. Hence, a volumetric titration of seawater with a strong acid yields a TA, which consists almost completely of carbonate alkalinity. Total alkalinity (TA) has been operationally defined by Dickson (1981):
where square brackets denote concentrations in gravimetric units (mol kg−1). The term “−[HNO2]” was later added by Dickson (Wolf-Gladrow et al., 2007). This expression is used/applied to measure TA by titration with a strong acid (HCl); therefore, it is also called titration alkalinity.
Wolf-Gladrow et al. (2007) combined Dickson's expression for TA with the concept of electroneutrality of aquatic solutions, to derive the “explicitly conservative” expression for TA. In a first step, the sum of concentrations of all ion, weighted by their charges, equals zero:
Next, the terms that show up in Dickson's TA expression are shifted to the right-hand side:
Add electrically neutral compounds (in bold) on both sides in order to obtain Dickson's TA expression on the right-hand side:
The right-hand side now equals Dickson's expression of TA. In a last step, sum up the species of the same acid-base system on the left-hand side (as the total concentrations are conservative):
where , , , and, , respectively. Again, the ellipses stand for additional, as yet unidentified, acid-base species. This expression is called “explicit conservative” because each single term is conservative in the sense that it does not change in a closed system with temperature or pressure and that they obey linear mixing relationships. Using TAec, it is easy to calculate the change of TA for dissolution or precipitation of CaCO3: TA changes by 2 moles per mol of CaCO3. Middelburg et al. (2020) argue that “it is important to distinguish between measurable titration alkalinity and charge balance alkalinity that is used to quantify calcification and carbonate dissolution and needed to understand the impact of biogeochemical processes on components of the carbon dioxide system.” While they refer to the ocean, the same is true for porewaters in an agricultural setting.
The contributions of other weak acid-base systems (the ellipses in Dickson's expression and in the TAec expression) are minor in open ocean seawater. However, in soil pore waters, rivers, and estuaries contributions by organic acids may contribute non negligible amounts to total alkalinity. The estimation of this organic alkalinity is a challenge because the chemical composition of the organic acids is usually not known and their pK-values vary over a wide pH range. Special titration methods (including back-titration with a strong base) have been proposed to obtain at least a rough estimate of the contribution of organic alkalinity (Wang and Cai, 2025). However, no routine analytical procedure has been developed for seawater thus far, let alone for soils.
The alkalinity in soils is very different from ocean alkalinity. This can be understood when comparing the oceanic with the terrestrial carbon cycle. In the ocean, organic matter synthesised in the photic zone by primary producers flows into an efficient food web, mostly within the mixed layer, where most of the carbon flows into higher trophic levels or is respired within the microbial loop. Faecal pellets, dead organisms and particulate organic matter sink to the ocean floor. This is called the biological pump because it pumps carbon against the DIC gradient into the deep ocean where almost all organic matter is respired to CO2. By contrast, in the terrestrial system, the organic biomass, and the decomposition thereof, remains in the soil matrix where “organic alkalinity8” and “nutrient alkalinity” play an important role. In comparison to the alkalinity in oceans, boron alkalinity in arable soils can be neglected as the majority of the world's agricultural soils contain only 5–30 ppm total boron9 (Kloke, 1980). Similar to seawater, the proton condition and pH0 for soils needs to be evaluated. Defining/choosing a single pK value applying to all acid-base systems in soils as a dividing point between proton acceptors and proton donors is not trivial as dissolved humic substances are a mixture of many molecules/compounds and therefore display a range of pK values (see e.g. Fig. 5 in Fukushima et al., 1995).
The charge contribution of dissolved humic substances, i.e. humic and fulvic acids, dominantly comes from carboxylic and phenolic functional groups (Ritchie and Perdue, 2003). These have average pK values of ca. 3.7±2.4 and ca. 12.5±1.8, respectively (Perdue et al., 1984). As a result, for a titration to a pH of 4.5, which is the endpoint in most titrations, humic acids could contribute between ca. 1.0–2.0 eq kg−1 and fulvic acids between ca. 1.5–2.5 eq kg−1 of negative charge, depending on initial soil pH and ionic strength of the soil solution (Milne et al., 2001). In agricultural soils, pore water DOC varies between 12 and 104 mg C L−1, with a median value of 33 mg C L−1 (De Troyer et al., 2014). Assuming 50 % of C in humic substances, this is equivalent to 66 mg DOM L−1. Recent work in two agricultural soils has shown that circa 83 % of pore water DOM is reactive, with the vast majority present as fulvic acids (Qin et al., 2024). This means that for a titration endpoint to pH=4.5, the charge contribution from fulvic acids is approximately 82.5–137.5 µeq L−1, while the charge contribution from humic acids is negligible. This back-of-the-envelope calculation shows that at an endpoint of pH 4.5, the contribution of dissolved humic substances, specifically fulvic acids, to alkalinity is likely to be substantial. While phenolic functional groups of humic substances are included in the “titration alkalinity”, (most of) the carboxylic functional groups are not. In addition, this calculation does not yet take into account the contribution of Low Molecular Weight Organic Acids (LMWOAs) that are commonly found in the rhizosphere. The bottom line is that agricultural soils are a source of organic alkalinity that, when exported and long-lived, can even be traced in coastal waters.
As argued above for seawater, charge balance is always obeyed in soils and positive charge of the cations released from EW of basalt has to be balanced by the dissociation of weak acids and bases. Hence, following the fate of the cations seems to be the method of choice to monitor alkalinity additions.
Here we highlight the impact of agricultural practices on the CDR of EW. Notably, liming and mineral and nitrogen fertilization.
Liming
The application of lime from limestone (CaCO3) adds two moles of alkalinity for one mole of DIC and therefore is an overall sink of carbon as the calcium will become charge balanced. However, if the lime is produced via calcination, which requires a lot of heat/energy, it will be an overall source of CO2. Hence, the impact depends on the origin. However, most aglime originates from limestone and only negligible amounts from calcination.
Depending on soil pH and the contribution of strong acids from nitrification of ammonium fertilisers, liming can act as a carbon source or sink (West and Mcbride, 2005; Oh and Raymond, 2006; Hamilton et al., 2007).
Fertilisation
The main nitrogen fertiliser in agriculture is urea, but in some instances artificial fertiliser is used. If charge is added with this fertiliser (e.g. NaNO3), the does not compete for cations released from basalt and its impact is neutral. However, most artificial N fertilisers are ammonium () based and produced via the Haber–Bosch reaction, responsible for 1.4 % of the global CO2 emissions and 1 % of the global energy consumption (Capdevila-Cortada, 2019). Such a fertiliser has a carbon-debt to start with and nitrification can turn into nitric acid (HNO3) where the will compete with carbonic acid for neutralising cations. Hence, one can argue that the impact of nitrogen fertilisation in agriculture is reducing the CDR potential of EW. In simple terms, adding acid to the soil, will drive CO2 production/evasion somewhere along the chain from field to the coastal ocean:
However, if lime is applied in addition to fertiliser, local charge balance should be considered as part of the CDR on the catchment scale. The overall carbon storage of the combined processes, relative to without liming, will be increased because the introduced Ca2+ will charge-balance nitrate export. Hence, acidity interacting with bicarbonate (from carbonate or silicate weathering) in the field avoids carbon degassing downstream because it has been removed by the weathering process. Note, that it will not avoid “additional” degassing due to nitric acid but we argue that it does not abate the CDR potential from EW. A similar argument was made by Bertagni and Porporato (2022). Based on integrated hydrological and biogeochemical theory they conclude that the alkalinization carbon-capture efficiency (ACE) across the aquatic continuum from land to ocean may vary significantly depending on pH but do not affect the final efficiency in the ocean.
Production of N2O results from incomplete denitrification, a process which can be substantial in grasslands, where water tables are high and anoxic conditions may prevail. N2O has a global warming potential per molecule that is nearly 300 times that of carbon dioxide over a 100 year time scale. This should be further investigated and accounted for in carbon equivalents. It has been suggested that EW may limit the efflux of N2O (Chiaravalloti et al., 2023) by enhancing plant N uptake. Complete denitrification produces alkalinity and charge-balancing this through the carbonate system will result in carbon removal. The net reaction is:
Even if the timescales over which this unfolds are uncertain, carbon removal will increase despite strong acid dissolution of feedstock. On longer timescales one can also argue that, once in the ocean, nitrogen will be incorporated in Redfield ratio into organic matter (106 carbon for 16 nitrogen), i.e. more than 6 carbon captured for each nitrogen!
Analogy to Terra Preta
Amazonian black earth or “terra preta” is typically associated with human occupation, but it is uncertain whether it was created intentionally. Interestingly, “terra preta” is not only characterised by its high organic carbon content and the presence of biochar but also by a high density of broken ceramic artefacts (e.g. Schmidt et al., 2023). Ancient Amazonians produced a lot of breakable pottery and the prevalence of pottery shards may be incidental and not part of a plan to improve soil fertility, but the practice of adding biochar and pottery shards to organic leftovers of manioc, cassava, corn, papaya and bananas, sequestered and stored carbon in the soil for centuries (Lima et al., 2002; Costa et al., 2004) and may demonstrate that broken pottery is an essential ingredient in terra preta analogous to clay minerals for building up SOC in arable soils (Rowley et al., 2021).
Interestingly, Anthony et al. (2025) show that the combined ground rock, compost, and biochar amendment had the greatest increases in soil C stocks over 3 years. This can be a coincidence but that composition is very similar to terra preta (except that pottery shards are replaced by crushed rock forming clay minerals). Could it be that the combination of organic amendments, biochar and crushed rock is also the best practice for CDR?
No data sets were used in this article.
This study was conceived by JB and he also drafted the initial version of the manuscript. All co-authors contributed and revised earlier versions of the manuscript. PPvS specifically helped with regard to developing the section on clay minerals, MH and SV had a major input on the soil organic carbon section, and DWG critically assisted with the conversion of carbon-based alkalinity to cation-based alkalinity.
At least one of the (co-)authors is a member of the editorial board of Biogeosciences. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
Jelle Bijma specifically wants to thank Dirk Paessler and Ralf Steffens for initiating the Carbon Drawdown Foundation, their hard and innovative work, both in the field and in the greenhouse, and for putting EW on the CDR map. Without their continued support, the research leading to the ideas developed in this paper would not have been possible. Jelle Bijma also wants to thank Ralph Koczwara (Hemmerbach/reverce), Klaus Gumpp (Alvacom/reverce) and Jan Lohse (reverce) for initiating “reverce” to tackle issues specific to up-scaling EW. Jelle Bijma wants to express his deep gratitude to the Paessler Family Foundation for their continued support over the last 5 years to carry out enhanced weathering experiments in the field and immerse into the science of enhanced rock weathering that finally led to the preparation of a first draft of the current manuscript.
Jelle Bijma received support from the Paessler Family Foundation over the last 5 years. Tim Jesper Suhrhoff received funding from the Swiss National Science Foundation (grant no. P500PN_210790) as well as the Yale Center for Natural Carbon Capture. The article processing charges for this open-access publication were covered by the Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung.
This paper was edited by Bertrand Guenet and reviewed by Isabel Montañez and one anonymous referee.
Almaraz, M., Bingham, N. L., Holzer, I. O., Geoghegan, E. K., Goertzen, H., Sohng, J., and Houlton, B. Z.: Methods for determining the CO2 removal capacity of enhanced weathering in agronomic settings, Frontiers in Climate, 4, https://doi.org/10.3389/fclim.2022.970429, 2022.
Amann, T., Hartmann, J., Struyf, E., de Oliveira Garcia, W., Fischer, E. K., Janssens, I., Meire, P., and Schoelynck, J.: Enhanced Weathering and related element fluxes – a cropland mesocosm approach, Biogeosciences, 17, 103–119, https://doi.org/10.5194/bg-17-103-2020, 2020.
Anthony, T. L., Jones, A. R., and Silver, W. L.: Supplementing Enhanced Weathering With Organic Amendments Accelerates the Net Climate Benefit of Soil Amendments in Rangeland Soils, AGU Advances, 6, e2024AV001480, https://doi.org/10.1029/2024AV001480, 2025.
Archer, D. and Maier-Reimer, E.: Effect of deep-sea sedimentary calcite preservation on atmospheric CO2 concentration, Nature, 367, 260–263, 1994.
Archer, D., Eby, M., Brovkin, V., Ridgwell, A., Cao, L., Mikolajewicz, U., Caldeira, K., Matsumoto, K., Munhoven, G., Montenegro, A., and Tokos, K.: Atmospheric Lifetime of Fossil Fuel Carbon Dioxide, Annual Review of Earth and Planetary Sciences, 37, 117–134, https://doi.org/10.1146/annurev.earth.031208.100206, 2009.
Bai, Y. and Cotrufo, M. F.: Grassland soil carbon sequestration: Current understanding, challenges, and solutions, Science, 377, 603–608, https://doi.org/10.1126/science.abo2380, 2022.
Basile-Doelsch, I., Amundson, R., Stone, W. E. E., Borschneck, D., Bottero, J. Y., Moustier, S., Masin, F., and Colin, F.: Mineral control of carbon pools in a volcanic soil horizon, Geoderma, 137, 477–489, https://doi.org/10.1016/j.geoderma.2006.10.006, 2007.
Beerling, D. J., Leake, J. R., Long, S. P., Scholes, J. D., Ton, J., Nelson, P. N., Bird, M., Kantzas, E., Taylor, L. L., Sarkar, B., Kelland, M., DeLucia, E., Kantola, I., Müller, C., Rau, G., and Hansen, J.: Farming with crops and rocks to address global climate, food and soil security, Nature Plants, 4, 138–147, https://doi.org/10.1038/s41477-018-0108-y, 2018.
Beerling, D. J., Kantzas, E. P., Lomas, M. R., Wade, P., Eufrasio, R. M., Renforth, P., Sarkar, B., Andrews, M. G., James, R. H., Pearce, C. R., Mercure, J.-F., Pollitt, H., Holden, P. B., Edwards, N. R., Khanna, M., Koh, L., Quegan, S., Pidgeon, N. F., Janssens, I. A., Hansen, J., and Banwart, S. A.: Potential for large-scale CO2 removal via enhanced rock weathering with croplands, Nature, 583, 242–248, https://doi.org/10.1038/s41586-020-2448-9, 2020.
Beerling, D. J., Epihov, D. Z., Kantola, I. B., Masters, M. D., Reershemius, T., Planavsky, N. J., Reinhard, C. T., Jordan, J. S., Thorne, S. J., Weber, J., Val Martin, M., Freckleton, R. P., Hartley, S. E., James, R. H., Pearce, C. R., DeLucia, E. H., and Banwart, S. A.: Enhanced weathering in the US Corn Belt delivers carbon removal with agronomic benefits, Proceedings of the National Academy of Sciences, 121, e2319436121, https://doi.org/10.1073/pnas.2319436121, 2024.
Bergaya, F., Lagaly, G., and Vayer, M.: Chapter 2.11 – Cation and Anion Exchange, in: Developments in Clay Science, edited by: Bergaya, F., and Lagaly, G., Elsevier, 333–359, https://doi.org/10.1016/B978-0-08-098259-5.00013-5, 2013.
Berner, R. A., Lasaga, A. C., and Garrels, R. M.: The Carbonate-silicate geochemical cycle and its effect on the atmospheric carbon dioxide over the past 100 million years, American Journal of Science, 283, 641–683, 1983.
Bertagni, M. B. and Porporato, A.: The Carbon-Capture Efficiency of Natural Water Alkalinization: Implications For Enhanced weathering, Science of The Total Environment, 838, 156524, https://doi.org/10.1016/j.scitotenv.2022.156524, 2022.
Blume, H. P., Welp, G., Brümmer, G. W., Thiele-Bruhn, S., Horn, R., Tippkötter, R., Kandeler, E., Kögel-Knabner, I., Kretzschmar, R., and Stahr, K.: Scheffer/Schachtschabel: Lehrbuch der Bodenkunde, Springer Berlin Heidelberg, ISBN 9783662499603, 2016.
Boito, L., Steinwidder, L., Rijnders, J., Berwouts, J., Janse, S., Niron, H., Roussard, J., Vienne, A., and Vicca, S.: Enhanced Rock Weathering Altered Soil Organic Carbon Fluxes in a Plant Trial, Global Change Biology, 31, e70373, https://doi.org/10.1111/gcb.70373, 2025.
Bramble, D. S. E., Schöning, I., Brandt, L., Poll, C., Kandeler, E., Ulrich, S., Mikutta, R., Mikutta, C., Silver, W. L., Totsche, K. U., Kaiser, K., and Schrumpf, M.: Land use and mineral type determine stability of newly formed mineral-associated organic matter, Communications Earth and Environment, 6, 415, https://doi.org/10.1038/s43247-025-02400-3, 2025.
Brantley, S. L., Shaughnessy, A., Lebedeva, M. I., and Balashov, V. N.: How temperature-dependent silicate weathering acts as Earth's geological thermostat, Science, 379, 382–389, https://doi.org/10.1126/science.add2922, 2023.
Brunner, C., Hausfather, Z., and Knutti, R.: Durability of carbon dioxide removal is critical for Paris climate goals, Communications Earth and Environment, 5, 645, https://doi.org/10.1038/s43247-024-01808-7, 2024.
Buck, H. J., Carton, W., Lund, J. F., and Markusson, N.: Why residual emissions matter right now, Nature Climate Change, 13, 351–358, https://doi.org/10.1038/s41558-022-01592-2, 2023.
Buss, W., Hasemer, H., Sokol, N. W., Rohling, E. J., and Borevitz, J.: Applying minerals to soil to draw down atmospheric carbon dioxide through synergistic organic and inorganic pathways, Communications Earth and Environment, 5, 602, https://doi.org/10.1038/s43247-024-01771-3, 2024.
Cai, W.-J., Wang, Y., and Hodson, R. E.: Acid-Base Properties of Dissolved Organic Matter in the Estuarine Waters of Georgia, USA, Geochimica et Cosmochimica Acta, 62, 473–483, https://doi.org/10.1016/S0016-7037(97)00363-3, 1998.
Calabrese, S., Wild, B., Bertagni, M. B., Bourg, I. C., White, C., Aburto, F., Cipolla, G., Noto, L. V., and Porporato, A.: Nano- to Global-Scale Uncertainties in Terrestrial Enhanced Weathering, Environ. Sci. Technol., 56, 15261–15272, https://doi.org/10.1021/acs.est.2c03163, 2022.
Calogiuri, T., Hagens, M., Van Groenigen, J. W., Wichern, F., Poetra, R. P., Rieder, L., Janssens, I. A., Hartmann, J., Neubeck, A., Niron, H., Singh, A., Vlaeminck, S. E., Vicca, S., and Vidal, A.: Alive and dead earthworms capture carbon during mineral weathering through different pathways, Communications Earth and Environment, 6, 851, https://doi.org/10.1038/s43247-025-02766-4, 2025a.
Calogiuri, T., Janssens, I., Vidal, A., Van Groenigen, J. W., Verdonck, T., Corbett, T., Hartmann, J., Neubeck, A., Niron, H., Poetra, R. P., Rieder, L., Servotte, T., Singh, A., Van Tendeloo, M., Vlaeminck, S. E., Vicca, S., and Hagens, M.: How earthworms thrive and drive silicate rock weathering in an artificial organo-mineral system, Applied Geochemistry, 180, 106271, https://doi.org/10.1016/j.apgeochem.2024.106271, 2025b.
Capdevila-Cortada, M.: Electrifying the Haber–Bosch, Nature Catalysis, 2, 1055–1055, https://doi.org/10.1038/s41929-019-0414-4, 2019.
Chen, Y., Ke, S., Yan, Y., Bo, G., and Zheng, H.: Effects of biochar on the accumulation of necromass-derived carbon, the physical protection and microbial mineralization of soil organic carbon, Critical Reviews in Environmental Science and Technology, 54, 39–67, https://doi.org/10.1080/10643389.2023.2221155, 2024.
Chiaravalloti, I., Theunissen, N., Zhang, S., Wang, J., Sun, F., Ahmed, A. A., Pihlap, E., Reinhard, C. T., and Planavsky, N. J.: Mitigation of soil nitrous oxide emissions during maize production with basalt amendments, Frontiers in Climate, 5, https://doi.org/10.3389/fclim.2023.1203043, 2023.
Clarkson, M. O., Larkin, C. S., Swoboda, P., Reershemius, T., Suhrhoff, T. J., Maesano, C. N., and Campbell, J. S.: A review of measurement for quantification of carbon dioxide removal by enhanced weathering in soil, Frontiers in Climate, 6, https://doi.org/10.3389/fclim.2024.1345224, 2024.
Costa, M., Kern, D., Helena, A., Pinto, E., and Trindade, J.: The ceramic artifacts in archaeological black earth (terra preta) from lower Amazon region, Brazil: Mineralogy, Acta Amazonica, 34, https://doi.org/10.1590/S0044-59672004000200004, 2004.
Dafnomilis, I., den Elzen, M., and van Vuuren, D.: Paris targets within reach by aligning, broadening and strengthening net-zero pledges, Communications Earth and Environment, 5, 48, https://doi.org/10.1038/s43247-023-01184-8, 2024.
Dellinger, M., Gaillardet, J., Bouchez, J., Calmels, D., Louvat, P., Dosseto, A., Gorge, C., Alanoca, L., and Maurice, L.: Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes, Geochimica et Cosmochimica Acta, 164, 71–93, https://doi.org/10.1016/j.gca.2015.04.042, 2015.
De Troyer, I., Merckx, R., Amery, F., and Smolders, E.: Factors Controlling the Dissolved Organic Matter Concentration in Pore Waters of Agricultural Soils, Vadose Zone Journal, 13, vzj2013.2009.0167, https://doi.org/10.2136/vzj2013.09.0167, 2014.
Dickson, A. G.: An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data., Deep-Sea Reserach, 28A, 609–623, 1981.
Dickson, A. G.: The development of the alkalinity concept in marine chemistry, Mar. Chem., 40, 49–63, 1992.
Dietzen, C. and Rosing, M. T.: Quantification of CO2 uptake by enhanced weathering of silicate minerals applied to acidic soils, International Journal of Greenhouse Gas Control, 125, 103872, https://doi.org/10.1016/j.ijggc.2023.103872, 2023.
Doi, A., Khosravi, M., Ejtemaei, M., Nguyen, T. A. H., and Nguyen, A. V.: Specificity and affinity of multivalent ions adsorption to kaolinite surface, Applied Clay Science, 190, 105557, https://doi.org/10.1016/j.clay.2020.105557, 2020.
Edelenbosch, O. Y., Hof, A. F., van den Berg, M., de Boer, H. S., Chen, H.-H., Daioglou, V., Dekker, M. M., Doelman, J. C., den Elzen, M. G. J., Harmsen, M., Mikropoulos, S., van Sluisveld, M. A. E., Stehfest, E., Tagomori, I. S., van Zeist, W.-J., and van Vuuren, D. P.: Reducing sectoral hard-to-abate emissions to limit reliance on carbon dioxide removal, Nature Climate Change, 14, 715–722, https://doi.org/10.1038/s41558-024-02025-y, 2024.
Farrah, H., Hatton, D., and Pickering, W. F.: The affinity of metal ions for clay surfaces, Chemical Geology, 28, 55–68, https://doi.org/10.1016/0009-2541(80)90035-2, 1980.
Friedlingstein, P., O'Sullivan, M., Jones, M. W., Andrew, R. M., Bakker, D. C. E., Hauck, J., Landschützer, P., Le Quéré, C., Luijkx, I. T., Peters, G. P., Peters, W., Pongratz, J., Schwingshackl, C., Sitch, S., Canadell, J. G., Ciais, P., Jackson, R. B., Alin, S. R., Anthoni, P., Barbero, L., Bates, N. R., Becker, M., Bellouin, N., Decharme, B., Bopp, L., Brasika, I. B. M., Cadule, P., Chamberlain, M. A., Chandra, N., Chau, T.-T.-T., Chevallier, F., Chini, L. P., Cronin, M., Dou, X., Enyo, K., Evans, W., Falk, S., Feely, R. A., Feng, L., Ford, D. J., Gasser, T., Ghattas, J., Gkritzalis, T., Grassi, G., Gregor, L., Gruber, N., Gürses, Ö., Harris, I., Hefner, M., Heinke, J., Houghton, R. A., Hurtt, G. C., Iida, Y., Ilyina, T., Jacobson, A. R., Jain, A., Jarníková, T., Jersild, A., Jiang, F., Jin, Z., Joos, F., Kato, E., Keeling, R. F., Kennedy, D., Klein Goldewijk, K., Knauer, J., Korsbakken, J. I., Körtzinger, A., Lan, X., Lefèvre, N., Li, H., Liu, J., Liu, Z., Ma, L., Marland, G., Mayot, N., McGuire, P. C., McKinley, G. A., Meyer, G., Morgan, E. J., Munro, D. R., Nakaoka, S.-I., Niwa, Y., O'Brien, K. M., Olsen, A., Omar, A. M., Ono, T., Paulsen, M., Pierrot, D., Pocock, K., Poulter, B., Powis, C. M., Rehder, G., Resplandy, L., Robertson, E., Rödenbeck, C., Rosan, T. M., Schwinger, J., Séférian, R., Smallman, T. L., Smith, S. M., Sospedra-Alfonso, R., Sun, Q., Sutton, A. J., Sweeney, C., Takao, S., Tans, P. P., Tian, H., Tilbrook, B., Tsujino, H., Tubiello, F., van der Werf, G. R., van Ooijen, E., Wanninkhof, R., Watanabe, M., Wimart-Rousseau, C., Yang, D., Yang, X., Yuan, W., Yue, X., Zaehle, S., Zeng, J., and Zheng, B.: Global Carbon Budget 2023, Earth Syst. Sci. Data, 15, 5301–5369, https://doi.org/10.5194/essd-15-5301-2023, 2023.
Friedlingstein, P., O'Sullivan, M., Jones, M. W., Andrew, R. M., Hauck, J., Landschützer, P., Le Quéré, C., Li, H., Luijkx, I. T., Olsen, A., Peters, G. P., Peters, W., Pongratz, J., Schwingshackl, C., Sitch, S., Canadell, J. G., Ciais, P., Jackson, R. B., Alin, S. R., Arneth, A., Arora, V., Bates, N. R., Becker, M., Bellouin, N., Berghoff, C. F., Bittig, H. C., Bopp, L., Cadule, P., Campbell, K., Chamberlain, M. A., Chandra, N., Chevallier, F., Chini, L. P., Colligan, T., Decayeux, J., Djeutchouang, L. M., Dou, X., Duran Rojas, C., Enyo, K., Evans, W., Fay, A. R., Feely, R. A., Ford, D. J., Foster, A., Gasser, T., Gehlen, M., Gkritzalis, T., Grassi, G., Gregor, L., Gruber, N., Gürses, Ö., Harris, I., Hefner, M., Heinke, J., Hurtt, G. C., Iida, Y., Ilyina, T., Jacobson, A. R., Jain, A. K., Jarníková, T., Jersild, A., Jiang, F., Jin, Z., Kato, E., Keeling, R. F., Klein Goldewijk, K., Knauer, J., Korsbakken, J. I., Lan, X., Lauvset, S. K., Lefèvre, N., Liu, Z., Liu, J., Ma, L., Maksyutov, S., Marland, G., Mayot, N., McGuire, P. C., Metzl, N., Monacci, N. M., Morgan, E. J., Nakaoka, S.-I., Neill, C., Niwa, Y., Nützel, T., Olivier, L., Ono, T., Palmer, P. I., Pierrot, D., Qin, Z., Resplandy, L., Roobaert, A., Rosan, T. M., Rödenbeck, C., Schwinger, J., Smallman, T. L., Smith, S. M., Sospedra-Alfonso, R., Steinhoff, T., Sun, Q., Sutton, A. J., Séférian, R., Takao, S., Tatebe, H., Tian, H., Tilbrook, B., Torres, O., Tourigny, E., Tsujino, H., Tubiello, F., van der Werf, G., Wanninkhof, R., Wang, X., Yang, D., Yang, X., Yu, Z., Yuan, W., Yue, X., Zaehle, S., Zeng, N., and Zeng, J.: Global Carbon Budget 2024, Earth Syst. Sci. Data, 17, 965–1039, https://doi.org/10.5194/essd-17-965-2025, 2025.
Frölicher, T. L., Fischer, E. M., and Gruber, N.: Marine heatwaves under global warming, Nature, 560, 360–364, https://doi.org/10.1038/s41586-018-0383-9, 2018.
Fukushima, M., Tanaka, S., Hasebe, K., Taga, M., and Nakamura, H.: Interpretation of the acid-base equilibrium of humic acid by a continuous pK distribution and electrostatic model, Analytica Chimica Acta, 302, 365–373, https://doi.org/10.1016/0003-2670(94)00471W, 1995.
Gaillardet, J., Dupré, B., Louvat, P., and Allègre, C. J.: Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers, Chemical Geology, 159, 3–30, https://doi.org/10.1016/S0009-2541(99)00031-5, 1999.
Ghabbour, E. A. and Davies, G.: Humic Substances: Structures, Models and Functions, The Royal Society of Chemistry, https://doi.org/10.1039/9781847551085, 2001.
Gislason, S., Arnorsson, S., and Ármannsson, H.: Chemical weathering of basalt in Southwest Iceland: Effects of runoff, age of rocks and vegetative/glacial cover, American Journal of Science, 296, 837–907, https://doi.org/10.2475/ajs.296.8.837, 1996.
Grimm, C., Martinez, R. E., Pokrovsky, O. S., Benning, L. G., and Oelkers, E. H.: Enhancement of cyanobacterial growth by riverine particulate material, Chemical Geology, 525, 143–167, https://doi.org/10.1016/j.chemgeo.2019.06.012, 2019.
Hagens, M. and Middelburg, J. J.: Generalised expressions for the response of pH to changes in ocean chemistry, Geochimica et Cosmochimica Acta, 187, 334–349, https://doi.org/10.1016/j.gca.2016.04.012, 2016.
Hamilton, S. K., Kurzman, A. L., Arango, C., Jin, L., and Robertson, G. P.: Evidence for carbon sequestration by agricultural liming, Global Biogeochemical Cycles, 21, https://doi.org/10.1029/2006GB002738, 2007.
Hansen, J. E., Sato, M., Simons, L., Nazarenko, L. S., Sangha, I., Kharecha, P., Zachos, J. C., von Schuckmann, K., Loeb, N. G., Osman, M. B., Jin, Q., Tselioudis, G., Jeong, E., Lacis, A., Ruedy, R., Russell, G., Cao, J., and Li, J.: Global warming in the pipeline, Oxford Open Climate Change, 3, https://doi.org/10.1093/oxfclm/kgad008, 2023.
Haque, F., Santos, R. M., Dutta, A., Thimmanagari, M., and Chiang, Y. W.: Co-Benefits of Wollastonite Weathering in Agriculture: CO2 Sequestration and Promoted Plant Growth, ACS Omega, 4, 1425–1433, https://doi.org/10.1021/acsomega.8b02477, 2019.
Haque, F., Möller, B., Sagina, S., Odhiambo, C., Ondolo, H., Thuo, N., Kamau, K., and Davies, S.: Agronomic Performance of Enhanced Rock Weathering in a Tropical Smallholder System: A Maize Trial in Kenya, CDRXIV, https://doi.org/10.70212/cdrxiv.2025410.v1, 2025.
Hartmann, J., West, A. J., Renforth, P., Köhler, P., De La Rocha, C. L., Wolf-Gladrow, D. A., Dürr, H. H., and Scheffran, J.: Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification, Reviews of Geophysics, 51, 113–149, https://doi.org/10.1002/rog.20004, 2013.
Hemingway, J. D., Rothman, D. H., Grant, K. E., Rosengard, S. Z., Eglinton, T. I., Derry, L. A., and Galy, V. V.: Mineral protection regulates long-term global preservation of natural organic carbon, Nature, 570, 228–231, https://doi.org/10.1038/s41586-019-1280-6, 2019.
Hemond, H. F.: Acid neutralizing capacity, alkalinity, and acid-base status of natural waters containing organic acids, Environmental Science and Technology, 24, 1486–1489, https://doi.org/10.1021/es00080a005, 1990.
Hooss, G., Voss, R., Hasselmann, K., Maier-Reimer, E., and Joos, F.: A nonlinear impulse response model of the coupled carbon cycle-climate system (NICCS), Climate Dynamics, 18, 189–202, https://doi.org/10.1007/s003820100170, 2001.
Hou, J., Qian, Y., Liu, G., and Dong, R.: The Influence of Temperature, pH and Ratio on the Growth and Survival of Earthworms in Municipal Solid Waste, Agricultural Engineering International: the CIGR Ejournal, Manuscript FP 04 014. Vol. VII, 2005.
IPCC: Summary for Policymakers, in: Climate Change 2022 – Mitigation of Climate Change: Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, 3–48, https://doi.org/10.1017/9781009157926.001, 2023.
Isson, T. T., Planavsky, N. J., Coogan, L. A., Stewart, E. M., Ague, J. J., Bolton, E. W., Zhang, S., McKenzie, N. R., and Kump, L. R.: Evolution of the Global Carbon Cycle and Climate Regulation on Earth, Global Biogeochemical Cycles, 34, e2018GB006061, https://doi.org/10.1029/2018GB006061, 2020.
Jones, M. T., Pearce, C. R., and Oelkers, E. H.: An experimental study of the interaction of basaltic riverine particulate material and seawater, Geochimica et Cosmochimica Acta, 77, 108–120, https://doi.org/10.1016/j.gca.2011.10.044, 2012.
Kalderon-Asael, B., Katchinoff, J. A. R., Planavsky, N. J., Hood, A. v. S., Dellinger, M., Bellefroid, E. J., Jones, D. S., Hofmann, A., Ossa, F. O., Macdonald, F. A., Wang, C., Isson, T. T., Murphy, J. G., Higgins, J. A., West, A. J., Wallace, M. W., Asael, D., and Pogge von Strandmann, P. A. E.: A lithium-isotope perspective on the evolution of carbon and silicon cycles, Nature, 595, 394–398, https://doi.org/10.1038/s41586-021-03612-1, 2021.
Kantzas, E. P., Val Martin, M., Lomas, M. R., Eufrasio, R. M., Renforth, P., Lewis, A. L., Taylor, L. L., Mecure, J.-F., Pollitt, H., Vercoulen, P. V., Vakilifard, N., Holden, P. B., Edwards, N. R., Koh, L., Pidgeon, N. F., Banwart, S. A., and Beerling, D. J.: Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom, Nature Geoscience, 15, 382–389, https://doi.org/10.1038/s41561-022-00925-2, 2022.
Kanzaki, Y., Planavsky, N. J., and Reinhard, C. T.: New estimates of the storage permanence and ocean co-benefits of enhanced rock weathering, PNAS Nexus, 2, https://doi.org/10.1093/pnasnexus/pgad059, 2023.
Kanzaki, Y., Planavsky, N., Zhang, S., Jordan, J., and Reinhard, C. T.: Soil cation storage as a key control on the timescales of carbon dioxide removal through enhanced weathering, ESS Open Archive [preprint], V.1, March 5, https://doi.org/10.22541/essoar.170960101.14306457/v1, 2024.
Kästner, M., Miltner, A., Thiele-Bruhn, S., and Liang, C.: Microbial Necromass in Soils – Linking Microbes to Soil Processes and Carbon Turnover, Frontiers in Environmental Science, 9, https://doi.org/10.3389/fenvs.2021.756378, 2021.
Kennedy, M. J. and Wagner, T.: Clay mineral continental amplifier for marine carbon sequestration in a greenhouse ocean, Proceedings of the National Academy of Sciences, 108, 9776–9781, https://doi.org/10.1073/pnas.1018670108, 2011.
Kennedy, M. J., Pevear, D. R., and Hill, R. J.: Mineral Surface Control of Organic Carbon in Black Shale, Science, 295, 657–660, https://doi.org/10.1126/science.1066611, 2002.
Kennedy, M. J., Löhr, S. C., Fraser, S. A., and Baruch, E. T.: Direct evidence for organic carbon preservation as clay-organic nanocomposites in a Devonian black shale; from deposition to diagenesis, Earth and Planetary Science Letters, 388, 59–70, https://doi.org/10.1016/j.epsl.2013.11.044, 2014.
Kerr, D. E., Brown, P. J., Grey, A., and Kelleher, B. P.: The influence of organic alkalinity on the carbonate system in coastal waters, Marine Chemistry, 237, 104050, https://doi.org/10.1016/j.marchem.2021.104050, 2021.
Kerr, D. E., Turner, C., Grey, A., Keogh, J., Brown, P. J., and Kelleher, B. P.: OrgAlkCalc: Estimation of organic alkalinity quantities and acid-base properties with proof of concept in Dublin Bay, Marine Chemistry, 251, 104234, https://doi.org/10.1016/j.marchem.2023.104234, 2023.
Klemme, A., Rixen, T., Müller, M., Notholt, J., and Warneke, T.: Destabilization of carbon in tropical peatlands by enhanced weathering, Communications Earth and Environment, 3, 212, https://doi.org/10.1038/s43247-022-00544-0, 2022.
Kloke, A.: Richtwerte '80, Orientierungsdaten für tolerierbare Gesamtgehalte einiger Elemente in Kulturböden, VDLUFA-Mitteilungen, 1–3, 1980.
Köhler, P., Hartmann, J., and Wolf-Gladrow, D. A.: Geoengineering potential of artificially enhanced silicate weathering of olivine, P. Natl. Acad. Sci. USA, 107, 20228–20233, https://doi.org/10.1073/pnas.1000545107, 2010.
Kotz, M., Levermann, A., and Wenz, L.: The economic commitment of climate change, Nature, 628, 551–557, https://doi.org/10.1038/s41586-024-07219-0, 2024.
Krause, A. J., Sluijs, A., van der Ploeg, R., Lenton, T. M., and Pogge von Strandmann, P. A. E.: Enhanced clay formation key in sustaining the Middle Eocene Climatic Optimum, Nature Geoscience, 16, 730–738, https://doi.org/10.1038/s41561-023-01234-y, 2023.
Kump, L. R. and Arthur, M. A.: Global Chemical Erosion during the Cenozoic: Weatherability Balances the Budgets, in: Tectonic Uplift and Climate Change, edited by: Ruddiman, W. F., Springer US, Boston, MA, 399–426 pp., https://doi.org/10.1007/978-1-4615-5935-1_18, 1997.
Kunz, W., Henle, J., and Ninham, B. W.: “Zur Lehre von der Wirkung der Salze” (about the science of the effect of salts): Franz Hofmeister's historical papers, Current Opinion in Colloid and Interface Science, 9, 19–37, https://doi.org/10.1016/j.cocis.2004.05.005, 2004.
Lamboll, R. D., Nicholls, Z. R. J., Smith, C. J., Kikstra, J. S., Byers, E., and Rogelj, J.: Assessing the size and uncertainty of remaining carbon budgets, Nature Climate Change, 13, 1360–1367, https://doi.org/10.1038/s41558-023-01848-5, 2023.
Larkin, C. S., Andrews, M. G., Pearce, C. R., Yeong, K. L., Beerling, D. J., Bellamy, J., Benedick, S., Freckleton, R. P., Goring-Harford, H., Sadekar, S., and James, R. H.: Quantification of CO2 removal in a large-scale enhanced weathering field trial on an oil palm plantation in Sabah, Malaysia, Frontiers in Climate, 4, https://doi.org/10.3389/fclim.2022.959229, 2022.
Lei, K., Bucka, F. B., Teixeira, P. P. C., Buegger, F., Just, C., and Kögel-Knabner, I.: Balancing Organic and Inorganic Carbon Dynamics in Enhanced Rock Weathering: Implications for Carbon Sequestration, Global Change Biology, 31, e70186, https://doi.org/10.1111/gcb.70186, 2025.
Lima, H., Schaefer, C., Mello, J., Gilkes, R., and Ker, J.: Pedogenesis and pre-Colombian land use of “Terra Preta Anthrosols” (“Indian black earth”) of Western Amazonia, Geoderma, 110, 1–17, https://doi.org/10.1016/S0016-7061(02)00141-6, 2002.
Linke, T., Oelkers, E. H., Dideriksen, K., Möckel, S. C., Nilabh, S., Grandia, F., and Gislason, S. R.: The geochemical evolution of basalt Enhanced Rock Weathering systems quantified from a natural analogue, Geochimica et Cosmochimica Acta, 370, 66–77, https://doi.org/10.1016/j.gca.2024.02.005, 2024a.
Linke, T., Oelkers, E. H., Möckel, S. C., and Gislason, S. R.: Direct evidence of CO2 drawdown through enhanced weathering in soils, Geochemical Perspectives Letters, 30, 7–12, https://doi.org/10.7185/geochemlet.2415, 2024b.
Linow, S., Bijma, J., Gerhards, C., Hickler, T., Kammann, C., Reichelt, F., and Scheffran, J.: Kurzimpuls – Perspektiven auf negative CO2-Emissionen, Diskussionsbeiträge der Scientists for Future, 12, 19, https://doi.org/10.5281/zenodo.7392348, 2022.
Liu, P. R. and Raftery, A. E.: Country-based rate of emissions reductions should increase by 80 % beyond nationally determined contributions to meet the 2 °C target, Communications Earth and Environment, 2, 29, https://doi.org/10.1038/s43247-021-00097-8, 2021.
Louvat, P. and Allègre, C. J.: Present denudation rates on the island of Réunion determined by river geochemistry: Basalt weathering and mass budget between chemical and mechanical erosions, Geochimica et Cosmochimica Acta, 61, 3645–3669, https://doi.org/10.1016/S0016-7037(97)00180-4, 1997.
Lubbers, I. M., van Groenigen, K. J., Fonte, S. J., Six, J., Brussaard, L., and van Groenigen, J. W.: Greenhouse-gas emissions from soils increased by earthworms, Nature Climate Change, 3, 187–194, https://doi.org/10.1038/nclimate1692, 2013.
Luderer, G., Vrontisi, Z., Bertram, C., Edelenbosch, O. Y., Pietzcker, R. C., Rogelj, J., De Boer, H. S., Drouet, L., Emmerling, J., Fricko, O., Fujimori, S., Havlík, P., Iyer, G., Keramidas, K., Kitous, A., Pehl, M., Krey, V., Riahi, K., Saveyn, B., Tavoni, M., Van Vuuren, D. P., and Kriegler, E.: Residual fossil CO2 emissions in 1.5–2 °C pathways, Nature Climate Change, 8, 626–633, https://doi.org/10.1038/s41558-018-0198-6, 2018.
Maher, K. and Chamberlain, C. P.: Hydrologic Regulation of Chemical Weathering and the Geologic Carbon Cycle, Science, 343, 1502–1504, https://doi.org/10.1126/science.1250770, 2014.
Maier-Reimer, E. and Hasselmann, K.: Transport and storage of CO2 in the ocean – an inorganic ocean-circulation carbon cycle model, Climate Dynamics, 2, 63–90, https://doi.org/10.1007/BF01054491, 1987.
Manning, D. and Theodoro, S.: Enabling food security through use of local rocks and minerals, The Extractive Industries and Society, 7, https://doi.org/10.1016/j.exis.2018.11.002, 2018.
Manning, D. A. C., de Azevedo, A. C., Zani, C. F., and Barneze, A. S.: Soil carbon management and enhanced rock weathering: The separate fates of organic and inorganic carbon, European Journal of Soil Science, 75, e13534, https://doi.org/10.1111/ejss.13534, 2024.
Matus, F., Rumpel, C., Neculman, R., Panichini, M., and Mora, M. L.: Soil carbon storage and stabilisation in andic soils: A review, Catena, 120, 102–110, https://doi.org/10.1016/j.catena.2014.04.008, 2014.
Meinshausen, M., Lewis, J., McGlade, C., Gütschow, J., Nicholls, Z., Burdon, R., Cozzi, L., and Hackmann, B.: Realization of Paris Agreement pledges may limit warming just below 2 °C, Nature, 604, 304–309, https://doi.org/10.1038/s41586-022-04553-z, 2022.
Middelburg, J. J., Soetaert, K., and Hagens, M.: Ocean Alkalinity, Buffering and Biogeochemical Processes, Reviews of Geophysics, 58, e2019RG000681, https://doi.org/10.1029/2019rg000681, 2020.
Milne, C. J., Kinniburgh, D. G., and Tipping, E.: Generic NICA-Donnan Model Parameters for Proton Binding by Humic Substances, Environmental Science and Technology, 35, 2049–2059, https://doi.org/10.1021/es000123j, 2001.
Moon, S., Chamberlain, C. P., and Hilley, G. E.: New estimates of silicate weathering rates and their uncertainties in global rivers, Geochimica et Cosmochimica Acta, 134, 257–274, https://doi.org/10.1016/j.gca.2014.02.033, 2014.
Needham, S., Worden, R., and Cuadros, J.: Sediment ingestion by worms and the production of bio-clays: A study of macrobiologically enhanced weathering and early diagenetic processes, Sedimentology, 53, 567–579, https://doi.org/10.1111/j.1365-3091.2006.00781.x, 2006.
Niron, H., Vienne, A., Frings, P., Poetra, R., and Vicca, S.: Exploring the synergy of enhanced weathering and Bacillus subtilis: A promising strategy for sustainable agriculture, Global Change Biology, 30, e17511, https://doi.org/10.1111/gcb.17511, 2024.
Oelkers, E. H., Butcher, R., Pogge von Strandmann, P. A. E., Schuessler, J. A., von Blanckenburg, F., Snæbjörnsdóttir, S. Ó., Mesfin, K., Aradóttir, E. S., Gunnarsson, I., Sigfússon, B., Gunnlaugsson, E., Matter, J. M., Stute, M., and Gislason, S. R.: Using stable Mg isotope signatures to assess the fate of magnesium during the in situ mineralisation of CO2 and H2S at the CarbFix site in SW-Iceland, Geochimica et Cosmochimica Acta, 245, 542–555, https://doi.org/10.1016/j.gca.2018.11.011, 2019.
Oh, N.-H. and Raymond, P. A.: Contribution of agricultural liming to riverine bicarbonate export and CO2 sequestration in the Ohio River basin, Global Biogeochemical Cycles, 20, https://doi.org/10.1029/2005GB002565, 2006.
Ou, Y., Iyer, G., Clarke, L., Edmonds, J., Fawcett, A. A., Hultman, N., McFarland, J. R., Binsted, M., Cui, R., Fyson, C., Geiges, A., Gonzales-Zuñiga, S., Gidden, M. J., Höhne, N., Jeffery, L., Kuramochi, T., Lewis, J., Meinshausen, M., Nicholls, Z., Patel, P., Ragnauth, S., Rogelj, J., Waldhoff, S., Yu, S., and McJeon, H.: Can updated climate pledges limit warming well below 2 °C?, Science, 374, 693–695, https://doi.org/10.1126/science.abl8976, 2021.
Paessler, D., Hammes, J., Steffens, R., and Smet, I.: Insights from monitoring leachate alkalinity, pCO2 and CO2 efflux of 400 weathering experiments over one year, Working paper V1.0., https://doi.org/10.13140/RG.2.2.14022.28485, 2024.
Paessler, D., Hammes, J., Smet, I., Steffens, R., Stöckel, A. A., and Hartmann, J.: Learnings from running the world's largest greenhouse EW experiment, https://doi.org/10.13140/RG.2.2.18184.74247, 2025.
Peplow, M.: Enzymes boost “rock weathering” to trap CO2 in soil, Nature Biotechnology, 42, 1326–1328, https://doi.org/10.1038/s41587-024-02380-3, 2024.
Perdue, E. M., Reuter, J. H., and Parrish, R. S.: A statistical model of proton binding by humus, Geochimica et Cosmochimica Acta, 48, 1257–1263, https://doi.org/10.1016/0016-7037(84)90060-7, 1984.
Pogge von Strandmann, P. A. E. and Henderson, G. M.: The Li isotope response to mountain uplift, Geology, 43, 67–70, https://doi.org/10.1130/g36162.1, 2015.
Pogge von Strandmann, P. A. E., Burton, K. W., James, R. H., van Calsteren, P., and Gislason, S. R.: Assessing the role of climate on uranium and lithium isotope behaviour in rivers draining a basaltic terrain, Chemical Geology, 270, 227–239, https://doi.org/10.1016/j.chemgeo.2009.12.002, 2010.
Pogge von Strandmann, P. A. E., Fraser, W. T., Hammond, S. J., Tarbuck, G., Wood, I. G., Oelkers, E. H., and Murphy, M. J.: Experimental determination of Li isotope behaviour during basalt weathering, Chemical Geology, 517, 34–43, https://doi.org/10.1016/j.chemgeo.2019.04.020, 2019.
Pogge von Strandmann, P. A. E., Renforth, P., West, A. J., Murphy, M. J., Luu, T.-H., and Henderson, G. M.: The lithium and magnesium isotope signature of olivine dissolution in soil experiments, Chemical Geology, 560, 120008, https://doi.org/10.1016/j.chemgeo.2020.120008, 2021.
Pogge von Strandmann, P. A. E., Liu, X., Liu, C.-Y., Wilson, D. J., Hammond, S. J., Tarbuck, G., Aristilde, L., Krause, A. J., and Fraser, W. T.: Lithium isotope behaviour during basalt weathering experiments amended with organic acids, Geochimica et Cosmochimica Acta, 328, 37–57, https://doi.org/10.1016/j.gca.2022.04.032, 2022a.
Pogge von Strandmann, P. A. E., Tooley, C., Mulders, J. J. P. A., and Renforth, P.: The Dissolution of Olivine Added to Soil at 4 °C: Implications for Enhanced Weathering in Cold Regions, Frontiers in Climate, 4, https://doi.org/10.3389/fclim.2022.827698, 2022b.
Pogge von Strandmann, P. A. E., Cosford, L. R., Liu, C.-Y., Liu, X., Krause, A. J., Wilson, D. J., He, X., McCoy-West, A. J., Gislason, S. R., and Burton, K. W.: Assessing hydrological controls on the lithium isotope weathering tracer, Chemical Geology, 642, 121801, https://doi.org/10.1016/j.chemgeo.2023.121801, 2023.
Pogge von Strandmann, P. A. E., He, X., Zhou, Y., and Wilson, D. J.: Comparing open versus closed system weathering experiments using lithium isotopes, Applied Geochemistry, 189, 106458, https://doi.org/10.1016/j.apgeochem.2025.106458, 2025.
Qin, Y., Groenenberg, J. E., Viala, Y., Alves, S., and Comans, R. N. J.: Optimizing multi-surface modelling of available cadmium as measured in soil pore water and salt extracts of soils amended with compost and lime: The role of organic matter and reactive metal, Science of The Total Environment, 957, 177769, https://doi.org/10.1016/j.scitotenv.2024.177769, 2024.
Ramos, E. J., Larsen, W. J., Hou, Y., Muñoz, S., Kemeny, P. C., Scheingross, J. S., Repasch, M. N., Hovius, N., Sachse, D., Ibarra, D. E., and Torres, M. A.: Competition or collaboration: Clay formation sets the relationship between silicate weathering and organic carbon burial in soil, Earth and Planetary Science Letters, 628, 118584, https://doi.org/10.1016/j.epsl.2024.118584, 2024.
Reershemius, T.: BioRender, https://BioRender.com/9vz62gz (last access: 22 December 2025), 2025.
Reershemius, T., Kelland, M. E., Jordan, J. S., Davis, I. R., D'Ascanio, R., Kalderon-Asael, B., Asael, D., Suhrhoff, T. J., Epihov, D. Z., Beerling, D. J., Reinhard, C. T., and Planavsky, N. J.: Initial Validation of a Soil-Based Mass-Balance Approach for Empirical Monitoring of Enhanced Rock Weathering Rates, Environmental Science and Technology, 57, 19497–19507, https://doi.org/10.1021/acs.est.3c03609, 2023.
Renforth, P.: The potential of enhanced weathering in the UK, International Journal of Greenhouse Gas Control, 10, 229–243, https://doi.org/10.1016/j.ijggc.2012.06.011, 2012.
Renforth, P. and Henderson, G.: Assessing ocean alkalinity for carbon sequestration, Reviews of Geophysics, 55, 636–674, https://doi.org/10.1002/2016RG000533, 2017.
Ripple, W. J., Wolf, C., Gregg, J. W., Rockström, J., Mann, M. E., Oreskes, N., Lenton, T. M., Rahmstorf, S., Newsome, T. M., Xu, C., Svenning, J.-C., Pereira, C. C., Law, B. E., and Crowther, T. W.: The 2024 state of the climate report: Perilous times on planet Earth, BioScience, https://doi.org/10.1093/biosci/biae087, 2024.
Ritchie, J. D. and Perdue, E. M.: Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter, Geochimica et Cosmochimica Acta, 67, 85–96, https://doi.org/10.1016/S0016-7037(02)01044-X, 2003.
Rosa, L. and Gabrielli, P.: Achieving net-zero emissions in agriculture: a review, Environmental Research Letters, 18, 063002, https://doi.org/10.1088/1748-9326/acd5e8, 2023.
Rowley, M. C., Grand, S., and Verrecchia, É. P.: Calcium-mediated stabilisation of soil organic carbon, Biogeochemistry, 137, 27–49, https://doi.org/10.1007/s10533-017-0410-1, 2018.
Rowley, M. C., Grand, S., Spangenberg, J. E., and Verrecchia, E. P.: Evidence linking calcium to increased organo-mineral association in soils, Biogeochemistry, 153, 223–241, https://doi.org/10.1007/s10533-021-00779-7, 2021.
Schleussner, C.-F., Ganti, G., Lejeune, Q., Zhu, B., Pfleiderer, P., Prütz, R., Ciais, P., Frölicher, T. L., Fuss, S., Gasser, T., Gidden, M. J., Kropf, C. M., Lacroix, F., Lamboll, R., Martyr, R., Maussion, F., McCaughey, J. W., Meinshausen, M., Mengel, M., Nicholls, Z., Quilcaille, Y., Sanderson, B., Seneviratne, S. I., Sillmann, J., Smith, C. J., Steinert, N. J., Theokritoff, E., Warren, R., Price, J., and Rogelj, J.: Overconfidence in climate overshoot, Nature, 634, 366–373, https://doi.org/10.1038/s41586-024-08020-9, 2024.
Schmidt, M. J., Goldberg, S. L., Heckenberger, M., Fausto, C., Franchetto, B., Watling, J., Lima, H., Moraes, B., Dorshow, W. B., Toney, J., Kuikuro, Y., Waura, K., Kuikuro, H., Kuikuro, T. W., Kuikuro, T., Kuikuro, Y., Kuikuro, A., Teixeira, W., Rocha, B., Honorato, V., Tavares, H., Magalhães, M., Barbosa, C. A., da Fonseca, J. A., Mendes, K., Alleoni, L. R. F., Cerri, C. E. P., Arroyo-Kalin, M., Neves, E., and Perron, J. T.: Intentional creation of carbon-rich dark earth soils in the Amazon, Science Advances, 9, eadh8499, https://doi.org/10.1126/sciadv.adh8499, 2023.
Schuiling, R. D.: Geochemical engineering: some thoughts on a new research field, Applied Geochemistry, 5, 251–262, https://doi.org/10.1016/0883-2927(90)90001L, 1990.
Schuiling, R. D. and Krijgsman, P.: Enhanced weathering: An effective and cheap tool to sequester CO2, Climatic Change, 74, 349–354, 2006.
Siegenthaler, U. and Sarmiento, J. L.: Atmospheric carbon dioxide and the ocean, Nature, 365, 119–125, 1993.
Sigurdsson, B. and Gudleifsson, B.: Impact of afforestation on earthworm populations in Iceland, Icelandic Agricultural Sciences, 26, 21–36, 2013.
Soetaert, K., Hofmann, A. F., Middelburg, J. J., Meysman, F. J. R., and Greenwood, J.: The effect of biogeochemical processes on pH, Marine Chemistry, 105, 30–51, 2007.
Sohng, J., Sokol, N. W., Whiteaker, S., Schmidt, R., Holzer, I., Goertzen, H., Peña, J., Houlton, B. Z., Montañez, I., O'Geen, A., and Scow, K.: Combining organic amendments with enhanced rock weathering shifts soil carbon storage in croplands, Science of The Total Environment, 998, 180179, https://doi.org/10.1016/j.scitotenv.2025.180179, 2025.
Sokol, N. W., Sohng, J., Moreland, K., Slessarev, E., Goertzen, H., Schmidt, R., Samaddar, S., Holzer, I., Almaraz, M., Geoghegan, E., Houlton, B., Montañez, I., Pett-Ridge, J., and Scow, K.: Reduced accrual of mineral-associated organic matter after two years of enhanced rock weathering in cropland soils, though no net losses of soil organic carbon, Biogeochemistry, 167, 989–1005, https://doi.org/10.1007/s10533-024-01160-0, 2024.
Song, S., Bellerby, R. G. J., Wang, Z. A., Wurgaft, E., and Li, D.: Organic Alkalinity as an Important Constituent of Total Alkalinity and the Buffering System in River-To-Coast Transition Zones, Journal of Geophysical Research: Oceans, 128, e2022JC019270, https://doi.org/10.1029/2022JC019270, 2023.
Song, X., Wang, P., Van Zwieten, L., Bolan, N., Wang, H., Li, X., Cheng, K., Yang, Y., Wang, M., Liu, T., and Li, F.: Towards a better understanding of the role of Fe cycling in soil for carbon stabilization and degradation, Carbon Research, 1, 5, https://doi.org/10.1007/s44246-022-00008-2, 2022.
Steinwidder, L., Boito, L., Frings, P. J., Niron, H., Rijnders, J., de Schutter, A., Vienne, A., and Vicca, S.: Beyond Inorganic C: Soil Organic C as a Key Pathway for Carbon Sequestration in Enhanced Weathering, Global Change Biology, 31, e70340, https://doi.org/10.1111/gcb.70340, 2025.
Stevenson, F. J.: Humus chemistry: genesis, composition, reactions, John Wiley & Sons, ISBN 0471594741, 1994.
Suhrhoff, T. J., Reershemius, T., Wang, J., Jordan, J. S., Reinhard, C. T., and Planavsky, N. J.: A tool for assessing the sensitivity of soil-based approaches for quantifying enhanced weathering: a US case study, Frontiers in Climate, 6, https://doi.org/10.3389/fclim.2024.1346117, 2024.
Swoboda, P., Döring, T. F., and Hamer, M.: Remineralizing soils? The agricultural usage of silicate rock powders: A review, Science of The Total Environment, 807, 150976, https://doi.org/10.1016/j.scitotenv.2021.150976, 2022.
te Pas, E. E. E. M., Chang, E., Marklein, A. R., Comans, R. N. J., and Hagens, M.: Accounting for retarded weathering products in comparing methods for quantifying carbon dioxide removal in a short-term enhanced weathering study, Frontiers in Climate, 6, https://doi.org/10.3389/fclim.2024.1524998, 2025.
Tipping, E.: WHAMC – A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances, Computers and Geosciences, 20, 973–1023, https://doi.org/10.1016/0098-3004(94)90038-8, 1994.
Ulfsbo, A., Kuliński, K., Anderson, L. G., and Turner, D. R.: Modelling organic alkalinity in the Baltic Sea using a Humic-Pitzer approach, Marine Chemistry, 168, 18–26, https://doi.org/10.1016/j.marchem.2014.10.013, 2015.
Venegas, R., Acevedo, J., and Treml, E.: Three decades of ocean warming impacts on marine ecosystems: A review and perspective, Deep Sea Research Part II: Topical Studies in Oceanography, 212, 105318, https://doi.org/10.1016/j.dsr2.2023.105318, 2023.
Vicca, S., Goll, D. S., Hagens, M., Hartmann, J., Janssens, I. A., Neubeck, A., Peñuelas, J., Poblador, S., Rijnders, J., Sardans, J., Struyf, E., Swoboda, P., van Groenigen, J. W., Vienne, A., and Verbruggen, E.: Is the climate change mitigation effect of enhanced silicate weathering governed by biological processes?, Global Change Biology, 28, 711–726, https://doi.org/10.1111/gcb.15993, 2022.
Vidal, A., Blouin, M., Lubbers, I., Capowiez, Y., Sanchez-Hernandez, J. C., Calogiuri, T., and van Groenigen, J. W.: Chapter One – The role of earthworms in agronomy: Consensus, novel insights and remaining challenges, in: Advances in Agronomy, edited by: Sparks, D. L., Academic Press, 1–78, https://doi.org/10.1016/bs.agron.2023.05.001, 2023.
Vienne, A., Poblador, S., Portillo-Estrada, M., Hartmann, J., Ijiehon, S., Wade, P., and Vicca, S.: Enhanced Weathering Using Basalt Rock Powder: Carbon Sequestration, Co-benefits and Risks in a Mesocosm Study With Solanum tuberosum, Frontiers in Climate, 4, https://doi.org/10.3389/fclim.2022.869456, 2022.
Vienne, A., Frings, P., Poblador, S., Steinwidder, L., Rijnders, J., Schoelynck, J., Vinduskova, O., and Vicca, S.: Earthworms in an enhanced weathering mesocosm experiment: Effects on soil carbon sequestration, base cation exchange and soil CO2 efflux, Soil Biology and Biochemistry, 199, 109596, https://doi.org/10.1016/j.soilbio.2024.109596, 2024.
Vienne, A., Frings, P., Rijnders, J., Suhrhoff, T. J., Reershemius, T., Poetra, R. P., Hartmann, J., Niron, H., Estrada, M. P., Steinwidder, L., Boito, L., and Vicca, S.: Weathering without inorganic CDR revealed through cation tracing, EGUsphere [preprint], https://doi.org/10.5194/egusphere-2025-1667, 2025.
Walker, J. C. G., Hays, P. B., and Kasting, J. F.: A negative feedback mechanism for the long-term stabilization of Earth's surface temperature, J. Geophys. Res., 86, 9776–9782, https://doi.org/10.1029/JC086iC10p09776, 1981.
Wang, Y., Yao, Z., Zhan, Y., Zheng, X., Zhou, M., Yan, G., Wang, L., Werner, C., and Butterbach-Bahl, K.: Potential benefits of liming to acid soils on climate change mitigation and food security, Global Change Biology, 27, 2807–2821, https://doi.org/10.1111/gcb.15607, 2021.
Wang, Z. A. and Cai, W.-J.: The inorganic carbon system across the land-to-ocean continuum, in: Treatise on Geochemistry, 3rd edn., edited by: Anbar, A. and Weis, D., Elsevier, Oxford, 111–144, https://doi.org/10.1016/B978-0-323-99762-1.00032-2, 2025.
Weil, R. R. and Brady, N. C.: The nature and properties of soils, 15th edn., Global edition, Pearson, Harlow, England, ISBN 9780133254488, 2017.
West, A. J., Galy, A., and Bickle, M.: Tectonic and climatic controls on silicate weathering, Earth and Planetary Science Letters, 235, 211–228, https://doi.org/10.1016/j.epsl.2005.03.020, 2005.
West, T. O. and McBride, A. C.: The contribution of agricultural lime to carbon dioxide emissions in the United States: dissolution, transport, and net emissions, Agriculture, Ecosystems and Environment, 108, 145–154, https://doi.org/10.1016/j.agee.2005.01.002, 2005.
Wilson, D. J., Pogge von Strandmann, P. A. E., White, J., Tarbuck, G., Marca, A. D., Atkinson, T. C., and Hopley, P. J.: Seasonal variability in silicate weathering signatures recorded by Li isotopes in cave drip-waters, Geochimica et Cosmochimica Acta, 312, 194–216, https://doi.org/10.1016/j.gca.2021.07.006, 2021.
Wolf-Gladrow, D. A., Zeebe, R. E., Klaas, C., Kortzinger, A., and Dickson, A. G.: Total alkalinity: The explicit conservative expression and its application to biogeochemical processes, Marine Chemistry, 106, 287–300, 2007.
Wu, Y., Peng, Z., Wang, X., Huang, J., Yang, L., and Liu, L.: Warmer Climate Reduces the Carbon Storage, Stability and Saturation Levels in Forest Soils, Earth's Future, 13, e2024EF004988, https://doi.org/10.1029/2024EF004988, 2025.
Xu, J., Liu, J., Fu, Q., Zhang, M., Guo, B., Li, H., Chen, X., and Qiu, G.: Soil carbon turnover and balance in the priming effects of basalt, montmorillonite, and kaolinite in a Luvisol soil, Journal of Soils and Sediments, 24, 732–743, https://doi.org/10.1007/s11368-023-03676-8, 2024.
Yan, Y., Dong, X., Li, R., Zhang, Y., Yan, S., Guan, X., Yang, Q., Chen, L., Fang, Y., Zhang, W., and Wang, S.: Wollastonite addition stimulates soil organic carbon mineralization: Evidences from 12 land-use types in subtropical China, Catena, 225, 107031, https://doi.org/10.1016/j.catena.2023.107031, 2023.
Zhang, B., Kroeger, J., Planavsky, N., and Yao, Y.: Techno-Economic and Life Cycle Assessment of Enhanced Rock Weathering: A Case Study from the Midwestern United States, Environmental Science and Technology, 57, 13828–13837, https://doi.org/10.1021/acs.est.3c01658, 2023.
Zhang, H.-M., Liang, Z., Li, Y., Chen, Z.-X., Zhang, J.-B., Cai, Z.-C., Elsgaard, L., Cheng, Y., van Groenigen, K. J., and Abalos, D.: Liming modifies greenhouse gas fluxes from soils: A meta-analysis of biological drivers, Agriculture, Ecosystems and Environment, 340, 108182, https://doi.org/10.1016/j.agee.2022.108182, 2022.
Zhang, K., Liu, Z., McCarl, B. A., and Fei, C. J.: Enhancing Agricultural Soil Carbon Sequestration: A Review with Some Research Needs, Climate, 12, 151, https://doi.org/10.3390/cli12100151, 2024.
Zhang, S., Reinhard, C. T., Liu, S., Kanzaki, Y., and Planavsky, N. J.: A framework for modeling carbon loss from rivers following terrestrial enhanced weathering, Environmental Research Letters, 20, 024014, https://doi.org/10.1088/1748-9326/ada398, 2025.
Zhang, W., Hendrix, P. F., Dame, L. E., Burke, R. A., Wu, J., Neher, D. A., Li, J., Shao, Y., and Fu, S.: Earthworms facilitate carbon sequestration through unequal amplification of carbon stabilization compared with mineralization, Nature Communications, 4, 2576, https://doi.org/10.1038/ncomms3576, 2013.
For “natural control of the atmospheric CO2 concentration” see Appendix A.
For an explanation of “alkalinity” see Appendix B.
Note that without the addition of lime, acidity would still react with natural alkalinity pools. On multi-year timescales lime addition may become neutral or even negative when the calcium is charge-balancing dissolved carbon (see Sect. 6.1)
Note that these are blog posts that have not formally been reviewed but as they describe the results from the largest greenhouse based long term EW experiment worldwide and are prepared with the help of scientists, we report them here.
Note that a change in TA does not lead to exactly the same amount of DIC change as suggested by simple stoichiometry (cf. the calculation in Köhler et al., 2010).
Note that low pH conditions are undesired in agriculture and usually antagonized with liming.
Note that only ca. 3.5 % of TA in open ocean surface water is due to “borate alkalinity”
Whether or not, and to what extent, organic acid-base systems contribute to titration alkalinity depends on the pK value of the organic substance!
In addition, pKa is above 9 at a salinity of 0.
- Abstract
- Introduction
- Enhanced Rock Weathering and agriculture
- Is alkalinity in soils different from alkalinity in the ocean?
- Impact of ERW on organic carbon cycling
- MRV in agricultural soils
- Fate of the cations
- Conclusion and outlook
- Appendix A: Natural atmospheric CO2 control in a nutshell
- Appendix B: The concept of (ocean) alkalinity in a nutshell
- Appendix C: Alkalinity in soils
- Appendix D: Impact of agricultural practices on the CDR of EW
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- Enhanced Rock Weathering and agriculture
- Is alkalinity in soils different from alkalinity in the ocean?
- Impact of ERW on organic carbon cycling
- MRV in agricultural soils
- Fate of the cations
- Conclusion and outlook
- Appendix A: Natural atmospheric CO2 control in a nutshell
- Appendix B: The concept of (ocean) alkalinity in a nutshell
- Appendix C: Alkalinity in soils
- Appendix D: Impact of agricultural practices on the CDR of EW
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References




